Photocatalytic adsorption-driven removal of rhodamine B by silver Tetratungstate-modified bentonite: Kinetic, isotherm, and thermodynamic analysis
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The influence of zwitterionic surfactant intercalation into photocatalyst-impregnated clay composites on textile dye removal has not been fully explored. In this study, three types of photoadsorbent composites: acid-activated bentonite (AB), a silver tetratungstate (Ag8W4O16) photocatalyst-doped version of AB known as Ag@AB, and a surfactant-intercalated variant called Ag@OAB, which incorporates dodecyl dimethyl betaine (BS12) were investigated on Rhodamine B (RhB) removal. The preparation of the AB composite involved treating natural bentonite with hydrochloric acid. The Ag@AB was created by depositing the photocatalyst into AB through a wet impregnation technique. Subsequently, BS12 was intercalated into the Ag@AB to form the Ag@OAB composite. The structural properties of these composites were analyzed using Scanning Electron Microscopy (SEM), X-Ray Diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR). The kinetics of Rhodamine B (RhB) removal by all three composites were best described by the pseudo-second-order kinetic model, suggesting that chemisorption is the main mechanism of removal, rather than photocatalytic. Adsorption isotherm analysis showed that the AB composite best fit the Langmuir model, while Ag@AB and Ag@OAB followed the Redlich-Peterson model. Thermodynamic analysis indicated that RhB adsorption onto all three composites takes place spontaneously as a physical adsorption process and is exothermic. Although kinetic data suggests that electrostatic attraction is a driving force behind chemisorption, isotherm and thermodynamic data indicate that physisorption is the dominant mechanism. This discrepancy may be due to the repulsive interactions between the zwitterionic functional groups of RhB and the positively charged surfaces of the composites, which could diminish overall electrostatic attraction. The addition of the zwitterionic surfactant BS12 in the Ag@OAB composite likely increases these repulsive interactions, leading to a lower adsorption capacity compared to AB. Overall, the findings underscore the intricate balance of attractive and repulsive forces and highlight the enhanced photocatalytic degradation involved in RhB removal by these composites.
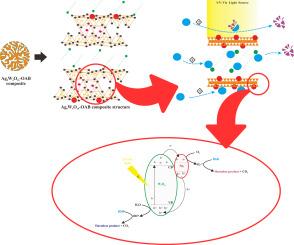
四钨酸银改性膨润土光催化吸附去除罗丹明B:动力学、等温线和热力学分析
两性离子表面活性剂嵌入光催化剂浸渍粘土复合材料对织物脱染性能的影响尚未得到充分的研究。在本研究中,研究了三种类型的光吸附复合材料:酸活化膨润土(AB),四钨酸银(Ag8W4O16)光催化剂掺杂的AB版本(Ag@AB)和表面活性剂插入的版本(Ag@OAB),其中包含十二烷基二甲基甜菜碱(BS12))对罗丹明B (RhB)的去除效果。AB复合材料的制备涉及用盐酸处理天然膨润土。Ag@AB是通过湿浸渍技术将光催化剂沉积到AB中而产生的。随后,将BS12插入到Ag@AB中,形成Ag@OAB复合材料。采用扫描电镜(SEM)、x射线衍射(XRD)和傅里叶变换红外光谱(FTIR)分析了复合材料的结构性能。三种复合材料对罗丹明B (Rhodamine B, RhB)的去除动力学用拟二级动力学模型描述得最好,表明化学吸附是主要的去除机理,而不是光催化。吸附等温线分析表明,AB复合材料最符合Langmuir模型,而Ag@AB和Ag@OAB符合Redlich-Peterson模型。热力学分析表明,RhB在三种复合材料上的吸附都是自发的物理吸附过程,并且是放热吸附。虽然动力学数据表明静电吸引是化学吸附背后的驱动力,但等温线和热力学数据表明物理吸附是主要机制。这种差异可能是由于RhB的两性离子官能团与复合材料的正电荷表面之间的排斥相互作用,这可能会降低整体静电吸引力。在Ag@OAB复合材料中加入两性离子表面活性剂BS12可能会增加这些排斥相互作用,导致与AB相比吸附能力较低。总的来说,研究结果强调了吸引力和排斥力的复杂平衡,并强调了这些复合材料在去除RhB过程中增强的光催化降解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


