Evolutionary history, longevity and terrestriality predict Toxoplasma gondii seroprevalence in free-ranging non-human primates
IF 2.2
3区 医学
Q3 ECOLOGY
International Journal for Parasitology-Parasites and Wildlife
Pub Date : 2025-09-30
DOI:10.1016/j.ijppaw.2025.101143
引用次数: 0
Abstract
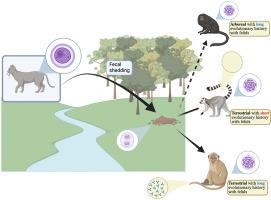
Evidence from captive populations indicates that lemurs are particularly vulnerable to toxoplasmosis, a parasitic disease caused by Toxoplasma gondii. In wild populations, however, seroprevalence in lemurs remains low. This may be partly due to their predominantly arboreal behavior, which limits exposure to environmentally transmitted oocysts. Alternatively, or additionally, low seroprevalence could reflect high mortality following infection due to limited evolutionary exposure to the parasite and, consequently, a lack of evolved resistance. In this study, we assess whether the evolutionary history of primates with felids influences susceptibility to T. gondii infection, independent of ecological exposure. Specifically, we predicted that (1) species with greater terrestriality would exhibit higher exposure risk, (2) species longevity would be positively associated with their seroprevalence to T. gondii and (3) primate superfamilies with longer histories of co-occurrence with felids would show higher seroprevalence than Lemuroidea at similar levels of terrestriality and longevity. Serum samples from 435 free-ranging lemurs were tested for T. gondii antibodies and a literature review of T. gondii seroprevalence in free-ranging primates was conducted. The overall seroprevalence in Lemuroidea was 5.4 %, significantly lower than that observed in Ceboidea (11.8 %) and Cercopithecoidea (27.6 %). Notably, seroprevalence in lemurs was lower than expected based on their terrestriality, suggesting that evolutionary isolation from felids may underlie heightened vulnerability. Longevity modifies the risk profile in a lineage-specific way where seroprevalence increases with lifespan in Cercopithecoidea but not for lemurs. Collectively, our findings support the hypothesis that lemurs are immunologically naïve to T. gondii, and in the face of expanding domestic cat populations and increasing habitat fragmentation, the parasite may pose an underrecognized conservation threat.
进化历史,寿命和陆地性预测刚地弓形虫在自由放养的非人灵长类动物中的血清流行率
来自圈养种群的证据表明,狐猴特别容易感染弓形虫病,这是一种由刚地弓形虫引起的寄生虫病。然而,在野生种群中,狐猴的血清患病率仍然很低。这可能部分是由于它们主要生活在树上,这限制了它们接触环境传播的卵囊。另外,低血清阳性率可能反映了感染后的高死亡率,这是由于有限的寄生虫进化暴露,因此缺乏进化抗性。在这项研究中,我们评估了灵长类动物与猫科动物的进化史是否会影响弓形虫感染的易感性,而不受生态暴露的影响。具体而言,我们预测:(1)陆生性较高的物种暴露风险较高;(2)物种寿命与其弓形虫血清阳性率呈正相关;(3)在相似的陆生性和寿命水平下,与猫科共发生历史较长的灵长类超科的血清阳性率高于狐猴总科。对435只散养狐猴的血清样本进行了刚地弓形虫抗体检测,并对散养狐猴中刚地弓形虫血清阳性率进行了文献综述。狐科总血清阳性率为5.4%,显著低于头科(11.8%)和尾科(27.6%)。值得注意的是,狐猴的血清阳性率低于基于其陆生性的预期,这表明与猫科动物的进化隔离可能是其脆弱性加剧的基础。长寿以一种特定谱系的方式改变了风险概况,在狐猴中,血清患病率随着寿命的增加而增加,而狐猴则没有。总的来说,我们的研究结果支持了狐猴对弓形虫免疫naïve的假设,并且面对家猫数量的增加和栖息地破碎化的加剧,寄生虫可能构成未被充分认识的保护威胁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal for Parasitology-Parasites and Wildlife
Medicine-Infectious Diseases
CiteScore
3.80
自引率
5.60%
发文量
113
审稿时长
45 days
期刊介绍:
The International Journal for Parasitology: Parasites and Wildlife (IJP-PAW) publishes the results of original research on parasites of all wildlife, invertebrate and vertebrate. This includes free-ranging, wild populations, as well as captive wildlife, semi-domesticated species (e.g. reindeer) and farmed populations of recently domesticated or wild-captured species (e.g. cultured fishes). Articles on all aspects of wildlife parasitology are welcomed including taxonomy, biodiversity and distribution, ecology and epidemiology, population biology and host-parasite relationships. The impact of parasites on the health and conservation of wildlife is seen as an important area covered by the journal especially the potential role of environmental factors, for example climate. Also important to the journal is ''one health'' and the nature of interactions between wildlife, people and domestic animals, including disease emergence and zoonoses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


