Transition metal-anchored WS2 nanosheets as efficient electrocatalysts for hydrogen evolution reaction: A first-principles study
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The development of efficient, low-cost, and stable electrocatalysts remains a central challenge for sustainable hydrogen production. In this work, we systematically designed a series of single transition metal (TM) atoms (including Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zr, Nb, Mo, Ru, Rh, Pd, Ag, Hf, Ta, W, Os, Ir, Pt, and Au) anchored on a WS2 monolayer, and investigated their catalytic activity for the hydrogen evolution reaction (HER) using density functional theory (DFT) calculations. The comprehensive results reveal that TM atoms can effectively tune the electronic structure, electrical conductivity, and hydrogen adsorption behavior of WS2. In particular, Cr@WS2, Fe@WS2, Mo@WS2, and Ru@WS2 catalysts exhibit Gibbs free energies of hydrogen adsorption close to zero, indicating their promising HER activity. Further crystal orbital Hamilton population (COHP) analyses and Bader charge calculations demonstrate that the chemical bonding characteristics and charge transfer between the TM atoms and the WS2 substrate play a pivotal role in tuning adsorption strength and catalytic performance. This work provides a theoretical foundation for optimizing WS2-based single-atom catalysts and opens new avenues for the design of highly efficient HER electrocatalysts based on two-dimensional materials.
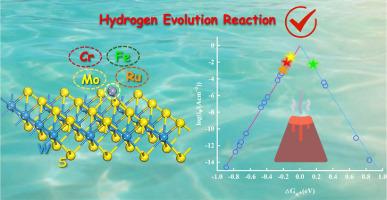
过渡金属锚定WS2纳米片作为析氢反应的高效电催化剂:第一线原理研究
开发高效、低成本、稳定的电催化剂仍然是可持续制氢的核心挑战。在这项工作中,我们系统地设计了一系列单一过渡金属(TM)原子(包括Ti、V、Cr、Mn、Fe、Co、Ni、Cu、Zr、Nb、Mo、Ru、Rh、Pd、Ag、Hf、Ta、W、Os、Ir、Pt和Au)锚定在WS2单层上,并使用密度泛函理论(DFT)计算研究了它们对析氢反应(HER)的催化活性。综合结果表明,TM原子可以有效调节WS2的电子结构、电导率和氢吸附行为。特别是Cr@WS2, Fe@WS2, Mo@WS2和Ru@WS2催化剂的氢吸附吉布斯自由能接近于零,表明它们具有良好的HER活性。进一步的晶体轨道汉密尔顿族(COHP)分析和Bader电荷计算表明,TM原子与WS2衬底之间的化学键特性和电荷转移在调节吸附强度和催化性能方面起着关键作用。本研究为优化基于ws2的单原子催化剂提供了理论基础,为设计基于二维材料的高效HER电催化剂开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


