PANI/Co3O4 composite material connected by coordination and hydrogen bonds for the elevation of rate charge/discharge capability
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
In this investigation, PANI/Co3O4 composite material with good rate charge/discharge capability was prepared through chemical grafting polyaniline (PANI) on the surface of homogeneous nucleated Co3O4. he physical properties were characterized by XRD, IR, XPS, SEM, EDS and N2 adsorption/desorption measurements. The charge/discharge performance was evaluated in 6 M KOH electrolyte using a symmetrical two-electrode configuration. The results show that Co3O4 has a face-centered cubic crystal structure with both Co2+ and Co3+ ions present in its lattice. After the modification, PANI particles covered the surface of Co3O4. Furthermore, PANI was bonded to Co3O4 via coordination and hydrogen bonds. Compared with Co3O4, the PANI/Co3O4 composite exhibits a 5.2 % increase in N content. Moreover, N atoms exist in PANI/Co3O4 as --, -NH- and = N- species. The PANI/Co3O4 composite has a specific surface area of 22 m2/g and a pore volume of 0.129 cm3/g, which are 1.57 times and 1.79 times those of pure Co3O4, respectively. In KOH electrolyte, the electrochemical behavior of PANI/Co3O4 is dominated by both surface control and ion diffusion processes. At a scan rate of 200 mV/s, the pseudocapacitive contribution of PANI/Co3O4 reaches 79.5 %, which is 3.2 % higher than that of Co3O4. Compared with Co3O4, PANI/Co3O4 displays a good rate charge/discharge performance. At 0.1 A/g, the energy density of PANI/Co3O4 is 6.7 Wh/kg. When the current density is enhanced to 5 A/g, the energy density retention is 43.4 %. This value is 1.18 times that of Co3O4, which is closely associated with the improved pore structure and the connection type between PANI and Co3O4.
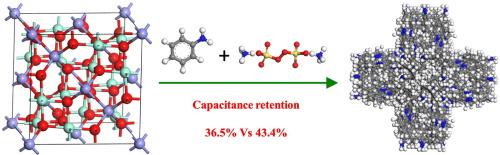
PANI/Co3O4复合材料通过配位和氢键连接,提高了倍率充放电能力
本研究通过在均质成核Co3O4表面接枝聚苯胺(PANI),制备了具有良好速率充放电性能的PANI/Co3O4复合材料。采用XRD、IR、XPS、SEM、EDS和N2吸附/脱附等手段对其进行了表征。在6 M KOH电解液中,采用对称双电极结构对其充放电性能进行了评价。结果表明:Co3O4具有面心立方晶体结构,其晶格中同时存在Co2+和Co3+离子;改性后,聚苯胺颗粒覆盖在Co3O4表面。此外,PANI通过配位键和氢键与Co3O4成键。与Co3O4相比,PANI/Co3O4复合材料的N含量增加了5.2%。此外,在PANI/Co3O4中,N原子以- nh +·-、- nh -和= N-的形式存在。PANI/Co3O4复合材料的比表面积为22 m2/g,孔体积为0.129 cm3/g,分别是纯Co3O4的1.57倍和1.79倍。在KOH电解液中,PANI/Co3O4的电化学行为主要受表面控制和离子扩散过程的控制。在200 mV/s的扫描速率下,PANI/Co3O4的赝电容贡献达到79.5%,比Co3O4高3.2个百分点。与Co3O4相比,PANI/Co3O4具有良好的倍率充放电性能。在0.1 A/g时,PANI/Co3O4的能量密度为6.7 Wh/kg。当电流密度增加到5 A/g时,能量密度保持率为43.4%。该值是Co3O4的1.18倍,这与改善的孔隙结构以及PANI与Co3O4的连接方式密切相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


