Interface adsorption enabled Ah-scale aqueous zinc-ion batteries
IF 7.9
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aqueous zinc-ion batteries (AZIBs) hold great potential for large-scale energy storage but are hindered by the limited reversibility of the Zn anode due to parasitic side reactions and uncontrolled dendrite growth. This study introduces ethylene diamine tetraacetic acid (EDTA) as an additive in aqueous electrolyte to lower the solvating capacity of water and promote anion coordination around Zn ions, thus accelerating desolvation kinetics. Stepwise theoretical calculations reveal that EDTA anion preferentially adsorbs on the Zn anode, effectively reshaping the electric double layer (EDL). Quantitative analysis using the Poisson−Boltzmann equation further demonstrates that the adsorption-type anionic additive increases Zn ion number concentration, reduces concentration polarization, and inhibits side reactions on the electrode surface, thereby enhancing charge transfer efficiency and optimizing the reaction process. With the addition of EDTA, Zn−V2O5 full cells with high mass loading (above 8 mg cm−2) exhibit improved self-discharge suppression, rate performance, and cycle performance. Notably, a successful transition from coin cells to larger-scale batteries has been demonstrated with a 1.21 Ah-scale pouch cell at the loading of 20 mg cm−2.
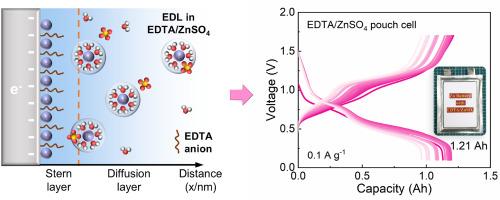
界面吸附使ah级水锌离子电池成为可能
水溶液锌离子电池(AZIBs)具有大规模储能的巨大潜力,但由于寄生副反应和不受控制的枝晶生长,锌阳极的可逆性有限,因此受到阻碍。本研究引入乙二胺四乙酸(EDTA)作为水溶液电解质添加剂,降低水的溶剂化能力,促进Zn离子周围阴离子配位,从而加快脱溶动力学。逐步理论计算表明,EDTA阴离子优先吸附在Zn阳极上,有效地重塑双电层(EDL)。利用泊松-玻尔兹曼方程进一步定量分析表明,吸附型阴离子添加剂增加了Zn离子数浓度,降低了浓度极化,抑制了电极表面的副反应,从而提高了电荷转移效率,优化了反应过程。添加EDTA后,具有高质量负载(大于8 mg cm−2)的Zn−V2O5全电池表现出更好的自放电抑制、倍率性能和循环性能。值得注意的是,从硬币电池成功过渡到更大规模的电池已经证明了1.21 ah规模的袋式电池在20 mg cm - 2的负载。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


