Effect of different anodes on the electrolytic process of molten salts
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-10-09
DOI:10.1016/j.jtice.2025.106460
引用次数: 0
Abstract
Background
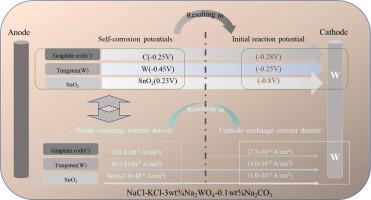
The use of different anode materials in the molten salt electrolysis process results in different products. Understanding the differences in electrochemical properties of different anodes during electrolysis is the basis for selecting the appropriate anode for molten salt systems. In this study, the differences in electrochemical behavior between graphite, tungsten rod and tin dioxide anodes were compared in NaCl-KCl-3 wt%Na2WO4–0.1 wt%Na2CO3 molten salt. The correlation between this variability and the deposition products was analyzed.
Methods
The differences in electrochemical behavior when using various anodes were analyzed in molten salts by means of potentiodynamic polarization curves, EIS spectra, linear scanning voltammetry curves, square wave voltammetry curves, open-circuit chronopotentiometry curves, and chronoamperometry curves tests.
Significant Findings
The potential difference exists between different anode materials during electrolysis. The larger the difference between the average exchange current densities of the anode and cathode, the more likely the cathode is to undergo a co-deposition reaction. The transient nucleation of cathode products is more favorable for the cathode interface co-deposition reaction. This provides an important reference value for the selection of anode in the molten salt electrolysis process.
不同阳极对熔盐电解过程的影响
在熔盐电解过程中使用不同的阳极材料会产生不同的产品。了解不同阳极在电解过程中电化学性能的差异,是为熔盐体系选择合适阳极的基础。本研究比较了在NaCl-KCl-3 wt% Na2WO4-0.1 wt%Na2CO3熔盐中石墨、钨棒和二氧化锡阳极的电化学行为差异。分析了这种变异性与沉积产物之间的相关性。方法采用动电位极化曲线、电阻抗光谱、线性扫描伏安曲线、方波伏安曲线、开路计时电位曲线和计时电流曲线试验,分析不同阳极在熔盐中电化学行为的差异。在电解过程中,不同阳极材料之间存在电位差。阳极和阴极的平均交换电流密度之差越大,阴极越有可能发生共沉积反应。阴极产物的瞬态成核更有利于阴极界面共沉积反应。这为熔盐电解过程中阳极的选择提供了重要的参考价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


