Synthesis and characterization of triazole-based schiff bases as novel highly efficient organocatalysts for rapid H2 generation via NaBH4 methanolysis
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
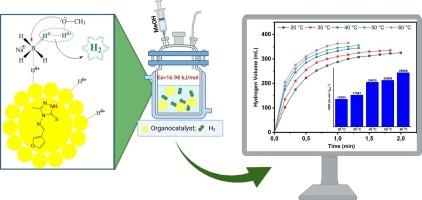
The triazole skeleton is a fundamental building block in heterocyclic chemistry and has been widely investigated for its applications in various fields such as materials science, medicinal chemistry and organic synthesis. Despite this broad utility, the use of this building group as an organocatalyst for hydrogen production is a largely neglected and under-researched topic in literature. While Schiff base–metal complexes (M = Cu, Zn, Ni, etc.) have previously been employed in sodium borohydride (NaBH4) hydrolysis reactions, it is particularly noteworthy that, for the first time, only triazole-based organocatalysts have been utilized as catalysts for hydrogen generation via the NaBH4 methanolysis. In this work, we synthesized, characterized, and optimized triazole-based organocatalysts (MF-MB-PF) and focused on as an efficient catalyst for hydrogen evolution via the alcoholysis of NaBH4. The hydrogen production performance of the MF catalyst was systematically evaluated on the basis of parameters such as catalyst type and amount used, NaBH4 concentration, different solvent systems, various methanol–water ratios, temperature conditions and catalyst reusability. The MF-catalyzed NaBH4 methanolysis reaction was completed in just 0.8 min. Moreover, the hydrogen generation rate (HGR) for the MF-catalyzed reaction was calculated to be 20,540 mL min−1gcat−1 at 30 ℃, which is comparable to, or better than, that of most metal-free catalysts used for similar purposes. In addition, we determined the activation energy for the MF-catalyzed reaction to be 16.98 kJ/mol. The MF catalyst exhibited remarkable reusability with no significant loss in the first three cycles in the catalytic activity used for the five-cycle reaction. Additionally, the possible mechanism of the MF catalyst in the NaBH4 methanolysis reaction is discussed. Overall, this study introduces MF as a novel metal-free catalyst for hydrogen generation and highlights its potential as an efficient material.
三唑基席夫碱作为NaBH4甲醇解快速制氢的新型高效有机催化剂的合成与表征
三唑骨架是杂环化学的基本组成部分,在材料科学、药物化学和有机合成等领域的应用得到了广泛的研究。尽管具有广泛的用途,但在文献中,将这种建筑群用作制氢的有机催化剂在很大程度上是一个被忽视和研究不足的话题。虽然希夫碱金属配合物(M = Cu, Zn, Ni等)之前已被用于硼氢化钠(NaBH4)水解反应,但特别值得注意的是,首次使用三唑基有机催化剂作为NaBH4甲醇解产氢的催化剂。在这项工作中,我们合成、表征和优化了三唑基有机催化剂(MF-MB-PF),并重点研究了它作为一种通过NaBH4醇解析氢的高效催化剂。根据催化剂种类和用量、NaBH4浓度、不同溶剂体系、不同甲醇水比、温度条件和催化剂的可重复使用性等参数,对MF催化剂的产氢性能进行了系统评价。mf催化的NaBH4甲醇分解反应在0.8 min内完成。此外,在30℃下,mf催化反应的氢生成速率(HGR)为20,540 mL min - 1gcat - 1,与大多数用于类似目的的无金属催化剂相当或更好。此外,我们确定了mf催化反应的活化能为16.98 kJ/mol。MF催化剂表现出显著的可重复使用性,在前三个循环中没有明显的催化活性损失,用于五循环反应。此外,还讨论了MF催化剂在NaBH4甲醇分解反应中的可能机理。总的来说,本研究介绍了MF作为一种新型的无金属制氢催化剂,并强调了其作为一种高效材料的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


