Native electrospray ionization mass spectrometry reveals the interaction variances between different bisphenol analogues and myoglobin
IF 4.2
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
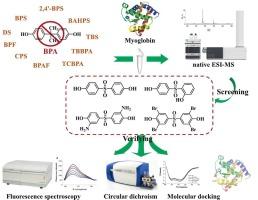
Bisphenol analogues (BPs) are crucial industrial materials extensively utilized in the production of consumer goods. However, it is still unknown whether they will affect the structure of myoglobin (Mb) and cause adverse impacts. This study systematically investigates the interactions between eleven BPs and their structural analogs with Mb using native electrospray ionization mass spectrometry (native ESI-MS), and further verified their interaction by fluorescence spectroscopy (FLS), circular dichroism (CD), and molecular docking (MD). Specifically, native ESI-MS analysis revealed that four ligands form stable complexes with Mb, and their binding constants were quantitatively determined. Thermodynamic data derived from FLS and CD analyses indicated that ligand binding induces conformational changes in Mb by reducing its α-helix content. MD simulations further elucidated that the binding sites for these ligands are located near the heme pocket of Mb, involving key interactions including hydrogen bonding and hydrophobic forces. Structure-activity relationship analysis suggested that sulfonyl and hydroxyl groups play critical roles in the ligand-Mb binding, where hydrophobic atoms enhance binding affinity by increasing molecular hydrophobicity, and ortho-substituted hydroxyl groups significantly influence binding strength. The integration approaches provide valuable information for the design and toxicity assessment of those bisphenol analogues in environmental implications.
原生电喷雾电离质谱法揭示了不同双酚类似物与肌红蛋白之间的相互作用差异
双酚类似物是广泛应用于消费品生产的重要工业材料。然而,它们是否会影响肌红蛋白(Mb)的结构并造成不良影响尚不清楚。本研究利用原生电喷雾质谱(ESI-MS)系统研究了11种bp及其结构类似物与Mb的相互作用,并通过荧光光谱(FLS)、圆二色性(CD)和分子对接(MD)进一步验证了它们的相互作用。具体来说,原生ESI-MS分析表明,四种配体与Mb形成稳定的配合物,并定量确定了它们的结合常数。从FLS和CD分析得到的热力学数据表明,配体结合通过降低其α-螺旋含量导致Mb构象发生变化。MD模拟进一步阐明了这些配体的结合位点位于Mb血红素口袋附近,涉及包括氢键和疏水力在内的关键相互作用。构效关系分析表明,磺酰基和羟基在配体- mb结合中起关键作用,其中疏水原子通过增加分子疏水性来增强结合亲和力,邻位取代羟基显著影响结合强度。这些综合方法为这些双酚类似物的环境影响设计和毒性评估提供了有价值的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


