Tailoring the Reaction Pathway of Glycerol Selective Oxidation by Constructing a Built-In Electric Field in Mott–Schottky Heterostructure Electrocatalyst Ni(OH)2–In
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Replacing the anodic oxygen evolution reaction with electro-oxidation of glycerol for hydrogen energy not only reduces energy consumption but also obtains high-value chemicals. However, this system still faces bottlenecks of limited Faradaic efficiency, low selectivity, and an unclear reaction pathway. Herein, the Mott–Schottky Ni(OH)2–In heterostructure was prepared for selective glycerol electro-oxidation (GOR). A series of experiments and theoretical calculations validated the charge shuttling through a heterointerface and the formation of the built-in electric field (BIEF) structure. As expected, the Ni(OH)2–In delivered a low potential of 1.22 V vs RHE at 10 mA cm–2. The Faradaic efficiency and selectivity for formate are up to 93% and 95%, respectively, together with long-term stability for the 20 cycles. The excellent performance can be attributed to the changed reaction pathway of producing the formate, reduced reaction energy barrier of the rate-determining step (RDS), and a more rapid GOR kinetics which arise from the robust BIEF. This work indicates that via constructing BIEF in the Mott–Schottky heterostructure, the electrocatalytic glycerol oxidation can be promoted.
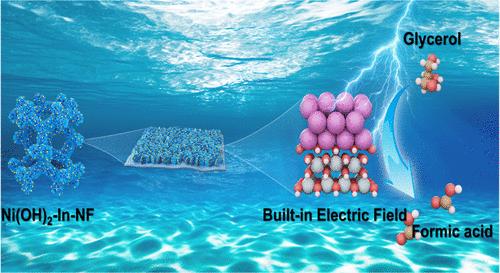
在Mott-Schottky异质结构电催化剂Ni(OH) 2-In中构建内置电场调整甘油选择性氧化反应途径
用甘油电氧化法代替阳极析氧反应制氢,不仅降低了能耗,而且获得了高价值的化学品。但该体系仍面临法拉第效率有限、选择性低、反应途径不明确等瓶颈。本文制备了用于选择性甘油电氧化(GOR)的Mott-Schottky Ni(OH) 2-In异质结构。一系列的实验和理论计算验证了电荷在异质界面上的穿梭和内置电场结构的形成。正如预期的那样,Ni(OH) 2-In在10 mA cm-2时提供了1.22 V vs RHE的低电位。对甲酸盐的选择性和法拉第效率分别达到93%和95%,并具有20次循环的长期稳定性。这种优异的性能可归因于改变了生成甲酸酯的反应途径,降低了速率决定步骤(RDS)的反应能垒,以及由于坚固的BIEF而产生的更快的GOR动力学。本研究表明,通过在Mott-Schottky异质结构中构建BIEF,可以促进电催化甘油氧化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


