Absolute Configuration of Key Intermediates and the Gram Scale Synthesis of Berotralstat and ent-Berotralstat
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Berotralstat (BCX-7353) is a potent plasma kallikrein inhibitor developed to prevent hereditary angioedema attacks. Initial racemic synthesis yielded limited quantities of the active (R)-enantiomer. To support clinical studies, a stereoselective synthetic route was developed. Key intermediates were isolated, and their stereochemistry confirmed via X-ray diffraction. These efforts enabled the scalable preparation of 16 g of berotralstat in 61% yield and nearly 1 g of its enantiomer in 23% yield─representing the first reported gram-scale synthesis of both compounds.
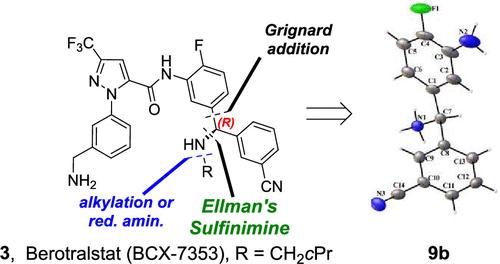
关键中间体的绝对构型及贝罗曲司他和正贝罗曲司他的克级合成
贝罗曲司他(BCX-7353)是一种有效的血浆钾likrein抑制剂,用于预防遗传性血管性水肿发作。最初的外消旋合成产生了有限数量的活性(R)-对映体。为了支持临床研究,我们开发了一种立体选择性合成路线。分离出了关键中间体,并通过x射线衍射证实了它们的立体化学性质。这些努力使16克贝曲司他的产量达到61%,近1克贝曲司他的对映体的产量达到23%,这是首次报道的克级合成这两种化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


