Solvent-Mediated C- or N-Center Selective Syn-Hydroheteroarylation of Ynamides with N-Heteroaromatics: Access to a Solvatofluorochromic, Mechanofluorochromic, and Thermochromic Material
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a solvent (HFIP)-mediated, C- or N-center selective, and 100% atom-economic syn-hydroheteroarylation of ynamides with N-heteroaromatics to access a wide variety of stereodefined α-heteroarylenamides at room temperature in good to excellent yield of up to 98%. While indole, pyrrole, and furan underwent the reaction with ynamides via a C-center, other N-heterocycles, such as pyrazole, triazole, and phenothiazine, underwent the same via the N-center, selectively. This chemo-, regio-, and stereoselective transformation required only HFIP, which played a couple of crucial roles, such as Brønsted acid to protonate ynamide regioselectively to generate the reactive keteniminium intermediate and then stabilize the intermediate as an indispensable solvent through H-bonding. Notably, the reactions were very clean, and the products were obtained pure without requiring column chromatography in many cases. HFIP is recovered through distillation (∼90% recovery) and recycled for subsequent reactions without any compromise in the reaction outcome. The green chemistry metrics of this protocol were excellent. Significantly, this green protocol enabled the direct synthesis of highly substituted N-H carbazoles from N-H indoles via a one-pot, two-step strategy by using only a couple of solvents, i.e., HFIP and toluene. Interestingly, a synthesized product, i.e., (Z)-N-(1-(1H-indol-3-yl)-2-(4-nitrophenyl)vinyl)-N-benzylbenzenesulfonamide, exhibited interesting properties, such as solvatofluorochromism, mechanofluorochromism, and reversible thermochromism.
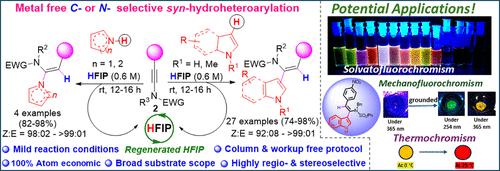
溶剂介导的与n-杂芳烃的酰胺的C-或n-中心选择性同步氢杂芳化:获得溶剂荧光变色、机械荧光变色和热变色材料
我们报道了一种溶剂(HFIP)介导的、C-或n-中心选择性的、100%原子经济的酰胺与n-杂芳烃的同步氢杂芳化反应,可以在室温下获得各种立体定义的α-杂芳酰胺,收率高达98%。吲哚、吡咯和呋喃通过c中心与酰胺发生反应,而其他n -杂环化合物,如吡唑、三唑和吩噻嗪,则选择性地通过n中心与酰胺发生反应。这种化学、区域和立体选择性转化只需要HFIP, HFIP发挥了几个关键作用,例如Brønsted酸对酰胺进行区域选择性质子化,生成反应性的氯胺中间体,然后通过氢键将中间体稳定为不可或缺的溶剂。值得注意的是,反应非常干净,在许多情况下,无需柱层析就能获得纯净的产品。HFIP通过蒸馏回收(回收率约90%),并在随后的反应中循环使用,而不会影响反应结果。该方案的绿色化学指标非常好。值得注意的是,这一绿色方案使N-H吲哚通过一锅两步策略直接合成高度取代的N-H咔唑,仅使用几种溶剂,即HFIP和甲苯。有趣的是,合成产物,即(Z)- n -(1-(1h -吲哚-3-基)-2-(4-硝基苯基)乙烯基)- n -苄基苯磺酰胺,表现出有趣的性质,如溶剂化荧光变色、机械荧光变色和可逆热变色。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


