Effect of Organic Vapors on the Behavior of Air Bubbles in Solutions of Nonionic and Ionic Surfactants
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
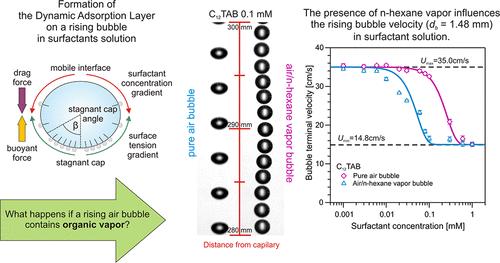
We explored the physicochemical aspects of the problem of a rising air bubble in an aqueous surfactant solution, where saturated n-hexane vapor is present within the bubble. The rising velocity profiles of these bubbles were measured in pure water and salt-free solutions of a nonionic (n-octanol) or cationic (dodecyltrimethylammonium bromide, C12TAB) surfactant at various concentrations. They were compared with the results for corresponding hexane-free systems. Additionally, dynamic surface tension for stationary bubbles was measured using bubble profile analysis tensiometry. To support these experimental data, we conducted an investigation using molecular dynamics (MD) simulations. For pure water, both surface tension measurements and MD simulations confirmed the adsorption of n-hexane molecules from the vapor phase to the stationary water interface, which is consistent with the literature reports. However, the rising bubble velocity was not affected by n-hexane vapor. We discuss this intriguing finding within the context of hydrodynamic forces. In the surfactant systems, a strong effect of coadsorption of surfactant from the solution and n-hexane from the vapor phase was observed in all investigations. The surface tension isotherms were theoretically described using a modified Frumkin adsorption model, additionally accounting for the ionic nature of C12TAB and the coadsorption of n-hexane from the vapor. The free energy of adsorption exhibited a strong correlation with the free energy profiles at the interface, as determined by MD simulations. The rising bubble data were theoretically analyzed in terms of the drag coefficient and the extent of bubble deformation. However, studies of the bubble velocity profiles revealed some unusual features, particularly during the dynamic layer formation phase.
有机蒸气对非离子和离子表面活性剂溶液中气泡行为的影响
我们探索了表面活性剂水溶液中气泡上升问题的物理化学方面,其中饱和正己烷蒸汽存在于气泡中。在纯水和不同浓度的非离子(正辛醇)或阳离子(十二烷基三甲基溴化铵,C12TAB)表面活性剂的无盐溶液中测量了这些气泡的上升速度曲线。并与相应的无己烷体系的结果进行了比较。此外,静态气泡的动态表面张力是用气泡轮廓分析张力测量法测量的。为了支持这些实验数据,我们使用分子动力学(MD)模拟进行了调查。对于纯水,表面张力测量和MD模拟都证实了正己烷分子从气相吸附到固定水界面,这与文献报道一致。而正己烷水蒸气对气泡上升速度没有影响。我们在水动力的背景下讨论这个有趣的发现。在表面活性剂体系中,在所有的研究中都观察到表面活性剂与气相正己烷的共吸附作用。采用改进的Frumkin吸附模型对表面张力等温线进行了理论描述,并考虑了C12TAB的离子性质和正己烷的共吸附。吸附的自由能与界面处的自由能分布有很强的相关性。从阻力系数和气泡变形程度两个方面对气泡上升数据进行了理论分析。然而,对气泡速度剖面的研究揭示了一些不寻常的特征,特别是在动态层形成阶段。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


