Conformational Analysis of Helical Peptides Incorporating Azepane-Based β-Amino Acids Prepared by an Ultrasound-Assisted Method
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report the synthesis of cis-5-aminoazepane-4-carboxylic acid (cis-AAzC) and its conformational behavior in nontraditional helical peptides. An ultrasound-assisted reductive amination method improves chemical yields and reduces solvent usage compared with previous methods. Incorporation of cis-AAzC into unnatural peptides promotes the 11/9-helix in 1:1 α/β-peptides and the 12/10-helix in β-peptides with enhanced aqueous solubility. The circular dichroism and the crystal structure data for these peptides suggest that additional functionalization at the azepane moiety is tolerated without disrupting helical propensity.
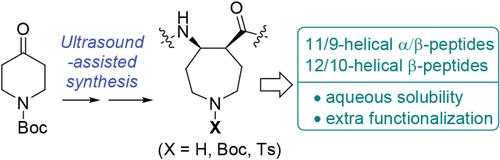
超声辅助法制备含氮杂烷基β-氨基酸的螺旋肽的构象分析
报道了顺-5-氨基氮杂烷-4-羧酸(cis-AAzC)的合成及其在非传统螺旋肽中的构象行为。与以前的方法相比,超声辅助还原性胺化方法提高了化学收率,减少了溶剂的使用。顺式aazc掺入非天然肽中,可促进1:1 α/β-肽中的11/9螺旋和β-肽中的12/10螺旋的水溶性增强。这些肽的圆二色性和晶体结构数据表明,在不破坏螺旋倾向的情况下,在氮烷部分的额外功能化是可以容忍的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


