Interaction between neighboring sulfur vacancies facilitates highly efficient nitrogen fixation in single-layer FeNiP2S6-x
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Iron-based transition metals, particularly iron-based thiophosphates, have attracted significant interest due to their ability to regulate iron electronic states through surface defect engineering. In this work, we fabricated and subsequently exfoliated single-layer FeNiP2S6-x (SL-FeNiP2S6-x), where mild and selective B(OH)3 etching generated a disordered distribution of surface sulfur vacancies (SVs) for efficient nitrogen reduction reactions (NRR). Single-layer theoretical calculations suggest that interactions between neighboring SVs significantly enhance nitrogen fixation, with single-double sulfur vacancy (S-DSV) interactions causing the most pronounced shift in the d-band center of iron atoms towards the Fermi level. The improved alignment between the production rates of *NN and *H indicates that S-DSV enhances the catalytic activity of FeNiP2S6-x more effectively than single-single sulfur vacancy (S-SSV) or double-double sulfur vacancy (D-DSV), and far outperforms isolated SSV or DSV. Electrochemical tests demonstrated that in a 0.1 M KOH solution at −0.1 V, SL-FeNiP2S6-x with a sufficient concentration (>50 %) of S-DSV achieved a Faraday efficiency (FE) of 51.30 % and an NH3 yield of 96.52 μg h−1 mg−1, surpassing the performance of previously reported NRR catalysts. This work introduces a useful strategy for enhancing electrocatalytic activity by strategically manipulating the position and distribution of adjacent non-metal atom vacancies on the surface.
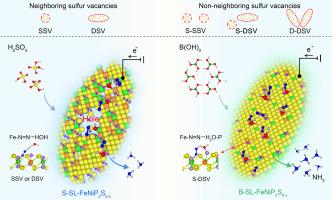
相邻硫空位之间的相互作用促进了单层FeNiP2S6-x的高效固氮
铁基过渡金属,特别是铁基硫代磷酸盐,由于其通过表面缺陷工程调节铁电子态的能力而引起了人们的极大兴趣。在这项工作中,我们制备并随后剥离了单层FeNiP2S6-x (SL-FeNiP2S6-x),其中轻度和选择性的B(OH)3蚀刻产生了无序分布的表面硫空位(sv),用于有效的氮还原反应(NRR)。单层理论计算表明,相邻sv之间的相互作用显著增强了固氮作用,其中单-双硫空位(S-DSV)相互作用导致铁原子d带中心向费米能级偏移最为明显。结果表明,S-DSV对FeNiP2S6-x的催化活性比单单硫空位(S-SSV)或双双硫空位(D-DSV)更有效,且远优于分离的SSV或DSV。电化学测试表明,在−0.1 M KOH、−0.1 V的条件下,当S-DSV浓度达到50 %时,SL-FeNiP2S6-x的法拉第效率(FE)为51.30 %,NH3产率为96.52 μg h−1 mg−1,优于之前报道的NRR催化剂的性能。这项工作介绍了一种有用的策略来提高电催化活性,通过策略性地操纵相邻非金属原子空位在表面上的位置和分布。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


