Soil protist diversity enhances prokaryotic diversity, and regulates dominant prokaryotes and the abundance of key nitrogen cycling genes
IF 10.3
1区 农林科学
Q1 SOIL SCIENCE
引用次数: 0
Abstract
Soil protists play crucial roles in soil microbial food-webs by preying on bacteria and other microorganisms. However, the effect of protist diversity on soil prokaryotic communities remains poorly understood. This study aimed to elucidate how different protist diversity treatments affect the composition and functionality of soil prokaryotic communities. We established soil microcosms with increasingly complex protist communities, including a control without protists, a medium diversity treatment with three small bacterivorous protists, and a high diversity treatment with seven protists of diverse trophic styles and sizes. Over 21 days, we monitored changes in the prokaryotic community using 16S rRNA gene sequencing and assessed the effects on nitrifiers and denitrifiers by qPCR of nitrogen-cycling genes. Protist diversity explained 23% of the observed prokaryotic community differentiation over time, with the high-diversity treatment causing the greatest divergence from the control. The most abundant prokaryotes were preferentially predated in all protist treatments. Unexpectedly, the absolute abundance of the nirK gene, which is widely distributed among bacterial taxa and thus associated with high functional redundancy, decreased. The differential response of genes with lower distribution and redundancy, such as the bacterial and archaeal amoA and the Nitrospira-associated nxrB genes, to protist diversity indicated selective predation on archaea. High protist diversity systematically enhanced these effects compared to the medium diversity treatment. Overall, protist diversity was positively associated with prokaryotic diversity, which is crucial for maintaining ecosystem stability. These findings highlight the critical role of protist diversity and likely complementary predation in shaping soil prokaryotic communities and their functioning, and open up new avenues to explore how this role may differ across soil types.
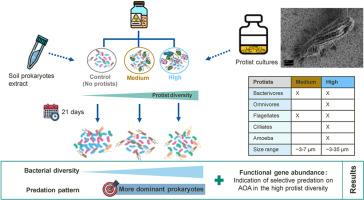
土壤原生生物多样性增强了原核生物多样性,调控了优势原核生物和氮循环关键基因的丰度
土壤原生生物以细菌和其他微生物为食,在土壤微生物食物网中起着至关重要的作用。然而,原生生物多样性对土壤原核生物群落的影响尚不清楚。本研究旨在阐明不同原生生物多样性处理对土壤原核生物群落组成和功能的影响。我们建立了原生生物群落日益复杂的土壤微观环境,包括不含原生生物的对照、含有3种小型细菌原生生物的中等多样性处理和含有7种不同营养类型和大小的原生生物的高多样性处理。在21天的时间里,我们通过16S rRNA基因测序监测了原核生物群落的变化,并通过氮循环基因的qPCR评估了对硝化菌和反硝化菌的影响。随着时间的推移,原生生物多样性解释了23%的观察到的原核生物群落分化,高多样性处理导致与对照的最大差异。在所有原生生物处理中,最丰富的原核生物优先被提前。出乎意料的是,广泛分布于细菌分类群并因此与高功能冗余相关的nirK基因的绝对丰度下降了。分布和冗余度较低的基因,如细菌和古细菌的amoA和硝化螺相关的nxrB基因,对原生生物多样性的差异反应表明了对古细菌的选择性捕食。与中等多样性处理相比,高原生生物多样性系统地增强了这些效果。总体而言,原生生物多样性与原核生物多样性呈正相关,这对维持生态系统的稳定至关重要。这些发现突出了原生生物多样性和可能的互补捕食在塑造土壤原核生物群落及其功能中的关键作用,并为探索这种作用在不同土壤类型中的差异开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Soil Biology & Biochemistry
农林科学-土壤科学
CiteScore
16.90
自引率
9.30%
发文量
312
审稿时长
49 days
期刊介绍:
Soil Biology & Biochemistry publishes original research articles of international significance focusing on biological processes in soil and their applications to soil and environmental quality. Major topics include the ecology and biochemical processes of soil organisms, their effects on the environment, and interactions with plants. The journal also welcomes state-of-the-art reviews and discussions on contemporary research in soil biology and biochemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


