Low volume expansion in S/O diatomically modified hard carbon for stable Na ion storage
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
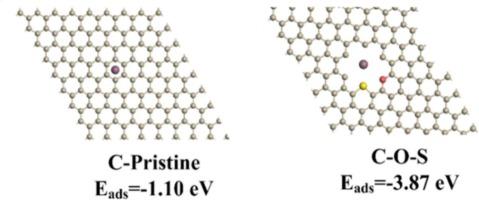
Sodium ion batteries (NIBs) show significant potential battery alternatives for lithium ion batteries (LIBs) large-scale energy grid storage. The development of cost-effective anodes for NIBs has become one of the most significant goals in contemporary society, which is characterized by high energy consumption. With merits of high abundance, sustainability and low cost, carbonaceous materials are utilized as highly promising NIB anodes. However, the commonly used carbon materials often suffer from large volume change upon Na+ ion intercalation/de-intercalation, leading to unsatisfactory capacity and poor cycling stability of NIBs. This study presents a chemical carbon etching strategy for the synthesis of sulfur (S)/oxygen (O) diatomically modified hard carbon with low volume expansion as a new type of anode for NIBs by the thermolysis of disused sunflower biomass. The S/O diatomically modified on the carbon matrix provides lower adsorption energy for sodium ion adsorption (−3.87 eV), effectively enhancing sodium storage capacity. Structural characterizations reveal that the S/O-modified hard carbon exhibits expanded interlayer spacing (0.392 nm) and large surface area per unit mass (227.7 m2 g−1). Density functional theory (DFT) simulations validate that the S/O diatomic modification on carbon promotes sodium cation adsorption and storage, and the volume expansion upon Na ion intercalation is only 2.5 %. Electrochemical measurements demonstrate that the hard carbon electrode provides superior charge–discharge retention (294 mAh g−1 at 0.03 A g−1) and exceptional long-term cycle stability (∼86 % preservation after 20,000 cycles at 5 A g−1). The practical cell was assembled by pairing this anode with a layered oxide cathode, showing highly sustainable capacity (129 mAh g−1) and exceptional long-term cycling stability (86 % after 100 cycles). In addition, the full cells are able to work in a broad operation temperature from −30 to 40 °C (138mAh g−1 at 40 °C and 110mAh g−1 at −30 °C). This synthesis route not only enhances the economic viability of biomass hard carbon materials but also highlights their environmental sustainability, which holds great promise for advancing improvement of high-efficiency NIBs.
低体积膨胀的S/O双原子改性硬碳用于稳定的Na离子储存
钠离子电池(NIBs)是锂离子电池(LIBs)大规模电网存储的重要潜在电池替代品。在高能耗的当代社会,开发高性价比的nib阳极已成为最重要的目标之一。碳质材料具有丰度高、可持续性好、成本低等优点,是极具发展前景的NIB阳极材料。然而,常用的碳材料在Na+离子插入/脱插过程中往往会发生较大的体积变化,导致nib的容量不理想,循环稳定性差。本研究提出了一种化学碳蚀刻策略,利用废弃向日葵生物质热解制备低体积膨胀的硫(S)/氧(O)双原子改性硬碳作为NIBs的新型阳极。碳基体上的S/O双原子修饰为钠离子吸附提供了较低的吸附能(- 3.87 eV),有效提高了钠的存储容量。结构表征表明,S/ o改性硬碳层间距扩大(0.392 nm),单位质量表面积增大(227.7 m2 g−1)。密度泛函数理论(DFT)模拟验证了碳上的S/O双原子修饰促进了钠离子的吸附和储存,而Na离子插入后的体积膨胀仅为2.5 %。电化学测量表明,硬碳电极具有优异的充放电保持性(在0.03 A g−1时为294 mAh g−1)和优异的长期循环稳定性(在5 A g−1下进行20,000次循环后保存~ 86% %)。实际电池是通过将该阳极与层状氧化物阴极配对组装而成的,具有高度可持续的容量(129 mAh g- 1)和出色的长期循环稳定性(100次循环后86 %)。此外,完整的电池能够在−30至40 °C的广泛工作温度下工作(40 °C时138mAh g−1,−30 °C时110mAh g−1)。该合成路线不仅提高了生物质硬碳材料的经济可行性,而且突出了其环境可持续性,这对推进高效nib的改进具有很大的希望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


