Encapsulated cells as an enzyme replacement therapy for metachromatic leukodystrophy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
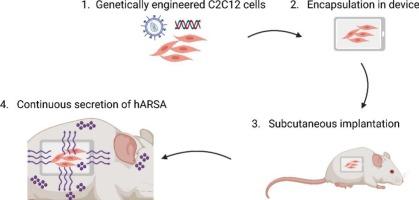
Metachromatic leukodystrophy (MLD) is a lysosomal storage disorder caused by an inherited deficiency of arylsulfatase A (ARSA) and characterized by accumulation of sulfatides in both glial cells and neurons resulting in myelin degeneration in the central and peripheral nervous systems, neuronal degeneration and neuroinflammation. Currently, no effective treatment is available for patients once symptoms are already present. Libmeldy©, an Ex vivo gene therapy, is the gold standard for pre-symptomatic patients with late-infantile and early juvenile MLD, and early symptomatic patients with early-juvenile MLD. Here, we investigated an innovative approach to deliver the recombinant ARSA enzyme by encapsulated-cell therapy. A cell-based device is capable of continuously delivering human ARSA compared to weekly intrathecal delivery of recombinant ARSA, which is under clinical evaluation in a phase II-III clinical trial. Two constructs of genetically engineered cells have been tested to determine which one could target more efficiently the central nervous system. Importantly, a dose response evaluation has been done with 2 doses (cells number loaded) to evaluate the best therapeutic effect. MLD mice were implanted at 6 months of age once disease is well established. The device biocompatibility as well as the therapeutic efficacy have been evaluated 3 months after implantation. The device was well tolerated in animals. Moreover, we demonstrate a correction of sulfatide storage and significant improvement of neuroinflammation in the CNS of all treated mice. All these data establish a strong rational to propose cell-based device therapy in symptomatic MLD patients.
包膜细胞作为一种酶替代疗法治疗异色性脑白质营养不良
异色性脑白质营养不良(MLD)是一种溶酶体贮积性疾病,由遗传性芳基硫酸酯酶a (ARSA)缺乏引起,其特征是神经胶质细胞和神经元中硫脂质的积累,导致中枢和周围神经系统髓磷脂变性、神经元变性和神经炎症。目前,一旦患者出现症状,就没有有效的治疗方法。Libmeldy©是一种离体基因疗法,是婴幼儿晚期和幼年早期MLD症状前患者和幼年早期MLD症状早期患者的金标准。在这里,我们研究了一种创新的方法,通过包封细胞治疗来传递重组ARSA酶。与每周鞘内给药重组ARSA相比,基于细胞的装置能够连续给药人ARSA,重组ARSA正在进行II-III期临床试验的临床评估。已经测试了两种基因工程细胞结构,以确定哪一种可以更有效地靶向中枢神经系统。重要的是,已经进行了2次剂量(细胞数量)的剂量反应评估,以评估最佳治疗效果。一旦疾病确定,MLD小鼠在6 个月大时被植入。植入3 个月后评价装置的生物相容性及治疗效果。这种装置在动物体内耐受性良好。此外,我们证明了所有治疗小鼠的中枢神经系统中硫脂储存的纠正和神经炎症的显着改善。这些数据为提出基于细胞的装置治疗症状性MLD患者提供了强有力的依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


