Applications of thianthrene chemistry in organic synthesis
IF 20
0 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
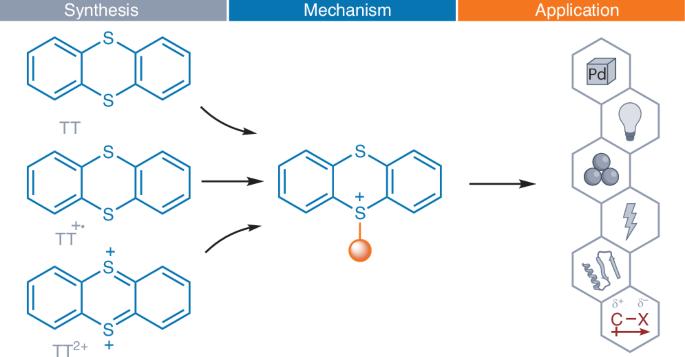
Thianthrene was first reported in 1869 and has been widely used in materials science for redox-flow batteries, polymers, supramolecular chemistry and phosphorescent materials. Despite extensive studies of the thianthrene radical cation and its reactivity since 1957, applications of thianthrene in synthetic chemistry were virtually absent from the literature until 2019. The then-discovered unusually high selectivity for thianthrenation by electrophilic aromatic substitution allowed for the synthesis of single constitutional isomers of structurally complex aryl-thianthrenium salts, which were mostly used as aryl (pseudo)halide analogues for subsequent functionalization. Since then, it has become apparent that the electronic structure of the thianthrenium substituent enables reaction chemistry that in part goes beyond what can be achieved with conventional organo(pseudo)halides. In this Review, we analyse and explain the fundamental aspects of organothianthrene chemistry, highlight the difference in reactivity to conventional organo(pseudo)halides and showcase its diverse applications. Thianthrene, long used in materials science, has recently emerged as a powerful reagent in organic synthesis. Its unique electronic structure enables access to diverse aryl, alkenyl and alkyl thianthrenium salts, which exhibit reactivity beyond conventional (pseudo)halides. This Review highlights the fundamental properties, distinctive reactivity and synthetic applications of these thianthrenium salts.
噻吩化学在有机合成中的应用
噻吩于1869年首次被报道,在材料科学中广泛应用于氧化还原液流电池、聚合物、超分子化学和磷光材料等领域。尽管自1957年以来对噻吩自由基阳离子及其反应性进行了广泛的研究,但直到2019年,文献中几乎没有关于噻吩在合成化学中的应用。随后发现的亲电芳取代对硫蒽化的高选择性使得芳基硫蒽盐的单构异构体的合成成为可能,这些异构体大多被用作芳基(伪)卤化物类似物,用于后续的功能化。从那时起,很明显,钍取代基的电子结构使反应化学在一定程度上超越了传统有机(伪)卤化物所能达到的水平。在这篇综述中,我们分析和解释了有机噻吩化学的基本方面,强调了与传统有机(伪)卤化物的反应性差异,并展示了它的多种应用。噻吩长期用于材料科学,最近在有机合成中成为一种强大的试剂。其独特的电子结构使其能够接触到各种芳基、烯基和烷基硫鎓盐,这些盐的反应性超过了传统的(伪)卤化物。本文综述了这些硫钍盐的基本性质、独特的反应性和合成应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


