Stable Electrothermal Reforming of Undiluted CH4/CO2 by Integrating Encapsulated Ni Nanoparticles with Internal Joule Heating
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Methane dry reforming (DRM) serves as a promising solution for mitigating carbon emission by valorizing two greenhouse gases (GHGs), methane and carbon dioxide, into value added syngas. However, the industrialization of DRM is practically hindered by extreme energy consumption, rapid coke formation, and severe metal sintering. All three limitations rooted in the high operation temperature required for overcoming the strong reaction endothermicity (ΔH = 247 kJ/mol). Herein, we presented an approach to address these bottlenecks via a highly efficient and stable electrothermal DRM (EDRM) process employing solely electricity-induced heat (Joule heating) as the heat source and adopting highly active structured Ni-embedded zeolite as catalysts. Using undiluted mixture of CH4 and CO2 as feedstock, EDRM exhibited superior space-time yield of syngas (17850 Lsyngas/(gNi h)) that surpassed the documented conventional DRM with Ni-based catalysts, together with a remarkable stability during 330 h time on stream at 800 °C without observable deactivation or coke deposition. The high activity (5.1 mmol/min) and stability were attributed to in-situ heat supply with high energy transfer efficiency (22.7%) and high resistance to temperature drop by reaction heat (less than ±3 °C), together with the embedding of Ni nanoparticles by zeolite that prevented metal from sintering. Meanwhile, mechanistic studies revealed that the high catalytic activity and stability of the Ni-embedded zeolite catalyst was achieved via a unique synergy between closely contacted silanol groups of the zeolite framework and embedded Ni particles that induced a carbonate-mediated pathway for efficient CO2 activation and rapid carbon consumption. This study not only presented a renewable energy-driven GHGs utilization strategy with industrial scalability but also highlighted the fundamental significance of electrothermal catalysis as an emerging interdisciplinary frontier bridging electrochemistry and thermal catalysis.
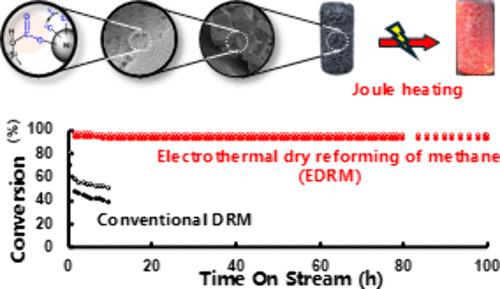
内焦耳加热集成封装Ni纳米颗粒对未稀释CH4/CO2的稳定电热重整
甲烷干式重整(DRM)通过将甲烷和二氧化碳两种温室气体(ghg)转化为增值合成气,是减少碳排放的一种有前途的解决方案。然而,DRM的产业化实际上受到能耗高、焦炭形成快、金属烧结严重等问题的阻碍。所有这三个限制都源于克服强反应吸热性所需的高操作温度(ΔH = 247 kJ/mol)。在此,我们提出了一种解决这些瓶颈的方法,即通过一种高效稳定的电热DRM (EDRM)工艺,该工艺仅采用电感应热(焦耳加热)作为热源,采用高活性结构镍嵌入沸石作为催化剂。使用未稀释的CH4和CO2混合物作为原料,EDRM表现出更高的时空合成气产率(17850 Lsyngas/(gNi h)),超过了使用ni基催化剂的传统DRM,并且在800°C下运行330 h时表现出出色的稳定性,没有观察到失活或积炭。该材料的高活性(5.1 mmol/min)和稳定性主要归功于原位热供给和高能量传递效率(22.7%)、高抗反应温度下降(小于±3℃)以及沸石包埋镍纳米颗粒防止金属烧结。同时,机理研究表明,镍包埋沸石催化剂的高催化活性和稳定性是通过沸石框架中紧密接触的硅醇基团与嵌入的Ni颗粒之间的独特协同作用实现的,这种协同作用诱导了碳酸盐介导的高效CO2活化和快速碳消耗途径。本研究不仅提出了一种具有工业可扩展性的可再生能源驱动的温室气体利用策略,而且强调了电热催化作为连接电化学和热催化的新兴跨学科前沿的基础意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


