Lewis acid sites modulate local pH of Cu for efficient electrocatalytic CO2 reduction to C2+ products
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Electrocatalytic carbon dioxide reduction reaction (CO2RR) offers a promising pathway to achieve carbon neutrality. For CO2RR, alkaline electrolytes not only suppress the hydrogen evolution reaction (HER) but also facilitate the formation of multi-carbon (C2+) products. However, alkaline electrolytes react with CO2 and continuously generate carbonates, which leads to poor carbon conversion and stability. Herein, CeO2 enriched with Lewis acid sites was incorporated into Cu catalyst to modulate local pH near Cu and promote C2+ formation in neutral electrolyte. In situ pH detection measurements including Raman spectroscopy and rotating ring-disk electrode demonstrate the elevated local pH at the vicinity of Cu induced by the addition of Lewis acid sites during CO2RR, while in situ spectroscopy combined with experiments design reveal the role of high local pH in promoting C2+ selectivity. Through this design for modulating local pH, CO2 is converted into C2+ products with high Faradaic efficiency (FEC2+) of 80.5 % at 750 mA cm−2, outperforming most reported Cu-based electrocatalysts in neutral electrolyte flow cells. This work provides a feasible approach to regulate the local microenvironment at the catalyst-electrolyte interface and stabilize key intermediates to enhance the generation of C2+ products.
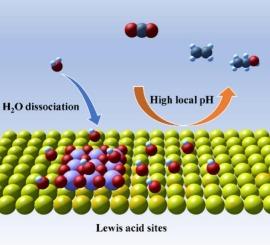
路易斯酸位点调节Cu的局部pH值,以有效地电催化CO2还原为C2+产物
电催化二氧化碳还原反应(CO2RR)为实现碳中和提供了一条很有前途的途径。对于CO2RR,碱性电解质不仅抑制了析氢反应(HER),而且促进了多碳(C2+)产物的形成。但碱性电解质与CO2反应,不断生成碳酸盐,导致碳转化率差,稳定性差。本文将富含Lewis酸位点的CeO2掺入Cu催化剂中,调节Cu附近的局部pH,促进中性电解质中C2+的生成。通过拉曼光谱和旋转环盘电极的原位pH检测表明,在CO2RR过程中,Lewis酸位点的加入导致Cu附近局部pH升高,而原位光谱结合实验设计揭示了高局部pH对C2+选择性的促进作用。通过这种调节局部pH值的设计,在750 mA cm−2下,CO2以80.5 %的高法拉第效率(FEC2+)转化为C2+产物,优于大多数报道的中性电解质流动电池中的cu基电催化剂。这项工作为调节催化剂-电解质界面的局部微环境和稳定关键中间体以促进C2+产物的生成提供了可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


