Ambipolar Ion Transport Membranes Enable Stable Noble-Metal-Free CO2 Electrolysis in Neutral Media
IF 26
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Carbon dioxide (CO2) electrolysis traditionally relies on monopolar ion-exchange membranes, including anion exchange membranes (AEM), cation exchange membranes (CEM), and a layered juxtaposition of two such monopolar membranes (bipolar membrane, BPM). The monopolar approach has worked well in water electrolysis in alkaline and acidic environments; however, in CO2 electrolysis, alkaline conditions promote carbonate formation, reducing stability, while acidic media favor competing hydrogen evolution reaction. Here, ambipolar ion transport membranes (AITMs) are reported in CO2 electrolysis. AITMs facilitate the free movement of ions and water between the cathode and anode, enabling stable CO2 electrolysis with highly concentrated electrolytes. This results in low operating cell voltages and compatibility with Earth-abundant anodes in pH-neutral electrolytes. Using a membrane electrode assembly cell, the electrochemical CO2 reduction is demonstrated in neutral media with a benchmark copper-sputtered polytetrafluoroethylene cathode and a nickel anode, achieving a high Faradaic efficiency of 75% for C2 products. This system operates at a current density of 110 mA cm−2 and a cell voltage of 3.15 V, maintaining a stable performance with a total operation exceeding 950 h. This work provides a pathway for stable CO2 conversion at relatively high current densities using Earth-abundant materials.
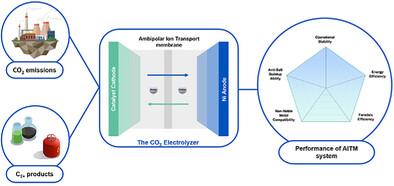
双极性离子传输膜在中性介质中实现稳定的无贵金属CO2电解
二氧化碳(CO2)电解传统上依赖于单极离子交换膜,包括阴离子交换膜(AEM),阳离子交换膜(CEM),以及两个单极膜(双极膜,BPM)的分层并置。单极子方法在碱性和酸性环境下的电解中效果良好;然而,在CO2电解中,碱性条件促进碳酸盐的形成,降低稳定性,而酸性介质有利于竞争性析氢反应。本文报道了双极性离子传输膜(AITMs)在CO2电解中的应用。aitm促进离子和水在阴极和阳极之间的自由运动,实现高浓度电解质稳定的CO2电解。这导致低工作电池电压和兼容性与地球丰富的阳极在ph中性电解质。利用膜电极组装电池,在中性介质中,以基准溅射铜聚四氟乙烯阴极和镍阳极进行了电化学CO2还原实验,C2产品的法拉第效率高达75%。该系统工作电流密度为110 mA cm - 2,电池电压为3.15 V,总工作时间超过950小时,保持稳定的性能。该工作为在相对高电流密度下使用地球上丰富的材料实现稳定的CO2转换提供了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


