Choline Iodide-Mediated Sulfur Conversion and Zinc Plating/Stripping Chemistry in Aqueous Zn–S Batteries
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous Zn–S batteries are promising candidates for future energy storage due to their intrinsic safety, environmental friendliness, and low cost. However, their practical application is hindered by sluggish sulfur redox kinetics and rapid zinc anode degradation. Here, we introduce choline iodide (ChI) as a multifunctional electrolyte additive that enables bidirectional catalysis of sulfur conversion and simultaneous protection of the zinc anode. During discharge, Ch+ promotes the formation of soluble polysulfide intermediates, which rapidly combine with Zn2+ to form ZnS via a solid–liquid–solid pathway, accelerating reaction kinetics. During charge, iodine species catalyze the conversion of ZnS back to sulfur. Moreover, Ch+ adsorbs on the zinc anode, suppressing dendrite growth and the hydrogen evolution reaction. Importantly, Ch+ also inhibits polyiodide shuttling at high iodine concentrations, maximizing catalytic efficiency. Coupled with a CoNC solid-phase catalyst, the Zn–S cell achieves a record-low polarization of 0.26 V at 0.1 C, delivers 780 mAh g−1 at 10 C, and maintains 380 mAh g−1 after 5500 cycles.
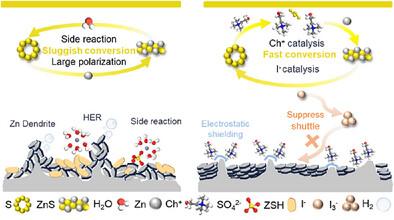
碘化胆碱介导的硫转化和水锌- s电池的镀锌/剥离化学
由于其固有的安全性、环保性和低成本,锌- s水溶液电池是未来能源存储的有希望的候选者。然而,它们的实际应用受到硫氧化还原动力学缓慢和锌阳极快速降解的阻碍。在这里,我们介绍了碘化胆碱(ChI)作为一种多功能电解质添加剂,可以双向催化硫转化并同时保护锌阳极。放电过程中,Ch+促进了可溶性多硫中间体的形成,这些中间体通过固-液-固途径与Zn2+快速结合形成ZnS,加速了反应动力学。在充电过程中,碘元素催化ZnS转化为硫。此外,Ch+吸附在锌阳极上,抑制枝晶生长和析氢反应。重要的是,Ch+还可以抑制高碘浓度下的多碘化物穿梭,从而最大化催化效率。与CoNC固相催化剂相结合,Zn-S电池在0.1 C时实现了0.26 V的创纪录低极化,在10 C时提供780 mAh g - 1,并在5500次循环后保持380 mAh g - 1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


