Ruthenium-Catalyzed Diversified Kinetic Resolutions of Diaryl-Substituted Cyclobutenones via Asymmetric Transfer Hydrogenation
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
1,2-Diaryl-3,4-dialkyl cyclobutanes (DA2CBs) are important scaffolds in numerous bioactive substances. However, the concise asymmetric synthesis of this type of compound remains a formidable challenge, especially in a stereodivergent manner. In this work, we report for the first time the diversified kinetic resolutions of diaryl-substituted cyclobutenones through Ru-catalyzed asymmetric transfer hydrogenation (ATH). The protocol affords a range of diaryl-substituted four-membered rings with structural and stereochemical diversity, which facilitates the total synthesis of six representative natural products in a stereodivergent fashion. Detailed mechanistic studies have been conducted to reveal the origin of the three kinetic resolution modes, and the catalysts are found to play vital roles in determining the reaction pathways, a phenomenon that has been scarcely observed in the field of ATH.
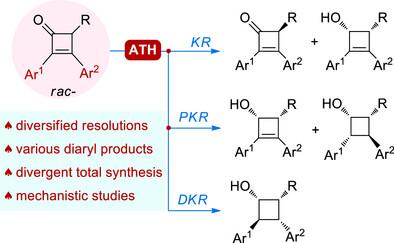
钌催化二芳基取代环丁烯酮不对称转移加氢的多种动力学拆分
1,2-二芳基-3,4-二烷基环丁烷(DA2CBs)是许多生物活性物质的重要支架。然而,这类化合物的简洁不对称合成仍然是一个巨大的挑战,特别是在立体发散的方式下。在这项工作中,我们首次报道了钌催化的不对称转移氢化反应(ATH)对二芳基取代环丁烯酮的不同动力学分辨率。该方案提供了一系列具有结构和立体化学多样性的二芳基取代四元环,这有助于以立体发散的方式合成六种具有代表性的天然产物。详细的机理研究揭示了三种动力学分解模式的起源,并发现催化剂在决定反应途径中起着至关重要的作用,这一现象在ATH领域几乎没有观察到。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


