Biodegradation pathways and mechanisms of perfluorooctanoic acid and perfluorooctanesulfonic acid via key enzymes in the marine microalga Chaetoceros calcitrans MZB-1
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Microalgae-mediated biodegradation of per- and poly-fluoroalkyl substances (PFASs) has gained significant attention. However, the degradation mechanism remains unclear, which is critical for optimizing biodegradation efficiency. This study aimed to elucidate the molecular mechanisms underlying perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) biodegradation by Chaetoceros calcitrans MZB-1 and reveal relevant degradation pathways. After cultivation for 15 days, the removal of PFOA and PFOS at initial concentration of 100 μg/L by alga MZB-1 was at 24.41 % and 29.16 %, respectively. Proteomics analysis identified significant upregulation of enzymes involved in PFOA/PFOS degradation, including cytochrome P450 monooxygenases (CYP450), haloacid dehalogenase, alkane monooxygenase, and taurine dioxygenase (TauD). Elucidation of the biodegradation mechanism revealed that PFOA underwent initial decarboxylation by CYP450 to form 1H-perfluoroheptane, followed by elimination, hydration, and isomerization reactions to generate 1 H,1H-perfluoroheptanol. This intermediate was further oxidized to the corresponding aldehydes and acids, ultimately producing perfluorohexanoic acid (PFHxA). For PFOS degradation, the process initiated with CF2 unit removal to form perfluoroheptanesulfonic acid (PFHpS), followed by TauD-mediated C-S bond cleavage and sulfonate group elimination at the end of carbon chain, converting PFHpS to 1H-perfluoroheptane. Then, the carbon chain gradually shortened, producing perfluoroheptanoic acid (PFHpA). Molecular docking showed that interactions between CYP450 and PFOA, as well as TauD and PFOS, primarily involved hydrogen bonding and hydrophobic interactions.
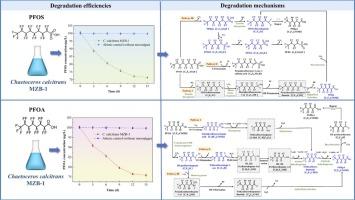
全氟辛酸和全氟辛烷磺酸在海洋微藻caetoceros calcitrans MZB-1中通过关键酶的生物降解途径和机制
微藻介导的全氟烷基和多氟烷基物质(PFASs)的生物降解已经引起了人们的广泛关注。然而,降解机制尚不清楚,这对优化生物降解效率至关重要。本研究旨在阐明calcitrans Chaetoceros MZB-1降解全氟辛酸(PFOA)和全氟辛烷磺酸(PFOS)的分子机制,揭示其降解途径。培养15 d后,MZB-1对PFOA和初始浓度为100 μg/L的PFOS的去除率分别为24.41%和29.16%。蛋白质组学分析发现,参与PFOA/PFOS降解的酶显著上调,包括细胞色素P450单加氧酶(CYP450)、卤酸脱卤酶、烷烃单加氧酶和牛磺酸双加氧酶(TauD)。对PFOA生物降解机理的研究表明,PFOA经过CYP450的初始脱羧反应生成1H-全氟庚烷,再经过消除、水化和异构化反应生成1H,1H-全氟庚烷醇。该中间体进一步氧化生成相应的醛和酸,最终生成全氟己酸(PFHxA)。对于全氟辛烷磺酸的降解,首先去除CF2单元形成全氟庚烷磺酸(PFHpS),然后通过taud介导的C-S键裂解和碳链末端的磺酸基消除,将PFHpS转化为1h -全氟庚烷。然后,碳链逐渐缩短,生成全氟庚酸(PFHpA)。分子对接表明,CYP450与PFOA、TauD与PFOS之间的相互作用主要是氢键和疏水相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


