Navel orange postharvest resistance towards Penicillium digitatum − role of histone demethylase CsJMJ12
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Histone methylation is essential for plant growth and development, and adaptation to biotic and abiotic stresses. However, its role in postharvest disease resistance in fruits remains poorly understood. The study aimed to investigate the involvement of histone demethylase CsJMJ12 in enhancing postharvest resistance to Penicillium digitatum in Navel orange (Citrus sinensis).Methods
Jumonji (JmjC) domain-containing proteins (JMJs) potentially involved in resistance to P. digitatum in Navel orange were identified using bioinformatics analysis, artificial inoculation and RT-qPCR. The role of JMJ12 in disease resistance and its underlying mechanism were further investigated through a series of assays, including subcellular location, Western blotting, immunofluorescence staining, transient overexpression, RNA-seq, widely targeted metabolomics, chromatin Immunoprecipitation (ChIP)-qPCR.Results
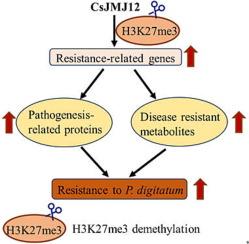
A total of 17 JMJs were identified from the citrus genome. In Navel orange infected with P. digitatum, the expression of CsJMJ12 was significantly upregulated. CsJMJ12 is localized to the nuclei and functions as a site-specific H3K27me3 histone demethylase. Overexpressing CsJMJ12 transiently in fruit resulted in increased resistance to P. digitatum, accompanied by reduced global H3K27me3 levels. Transcriptome sequencing revealed that CsJMJ12 regulates multiple defense pathways, including phenylpropanoid biosynthesis, lignin biosynthesis, cell wall organization, pathogenesis-related protein, and hormone signaling. Metabolomics showed that CsJMJ12 regulates the accumulation of disease-resistant metabolites such as dihydrocoumarin, caffeoylputrescine, prenylnaringenin, quinic acid and syringic acid in phenylpropanoid biosynthesis pathways. Furthermore, CsJMJ12 promoted the expression of key defense-associated genes, including POD, COMT1, LAC7L, SAME, SAMT, CHI, GT2 and CSL5, by demethylating H3K27me3 at their chromatin loci, thereby enhancing fruit resistance to P. digitatum.Conclusions
This study identifies CsJMJ12 as a potential epigenetic regulator of P. digitatum resistance in Navel orange. Our findings provide valuable insights into the molecular mechanism of pathogen defense in sweet orange (Citrus sinensis). Further studies are needed to explore its broader application in agricultural practices and postharvest disease management.
脐橙采后对指状青霉的抗性——组蛋白去甲基酶CsJMJ12的作用
组蛋白甲基化对植物生长发育以及适应生物和非生物胁迫至关重要。然而,它在果实采后抗病中的作用仍然知之甚少。本研究旨在探讨组蛋白去甲基酶CsJMJ12在提高脐橙采后对指状青霉抗性中的作用。方法采用生物信息学分析、人工接种和RT-qPCR等方法,对脐橙中可能与指状线虫抗性相关的巨onji结构域蛋白(JMJs)进行鉴定。通过亚细胞定位、Western blotting、免疫荧光染色、瞬时过表达、RNA-seq、广泛靶向代谢组学、染色质免疫沉淀(ChIP)-qPCR等一系列检测,进一步研究JMJ12在抗病性中的作用及其潜在机制。结果从柑橘基因组中共鉴定出17个JMJs。脐橙感染指状地黄后,CsJMJ12的表达显著上调。CsJMJ12定位于细胞核,作为位点特异性H3K27me3组蛋白去甲基化酶发挥作用。在果实中短暂过表达CsJMJ12会导致对地黄的抗性增强,同时降低全球H3K27me3水平。转录组测序显示,CsJMJ12调节多种防御途径,包括苯丙素生物合成、木质素生物合成、细胞壁组织、发病相关蛋白和激素信号传导。代谢组学研究表明,CsJMJ12调控了苯丙类生物合成途径中抗病代谢物如二氢香豆素、咖啡基腐胺、丙烯基柚皮素、奎宁酸和丁香酸的积累。此外,CsJMJ12通过在H3K27me3的染色质位点上去甲基化,促进POD、COMT1、LAC7L、SAME、SAMT、CHI、GT2和CSL5等关键防御相关基因的表达,从而增强果实对digitatum的抗性。结论CsJMJ12可能是脐橙对指地黄病菌抗性的表观遗传调控因子。本研究结果为甜橙病原菌防御的分子机制提供了有价值的见解。需要进一步的研究来探索其在农业实践和采后病害管理中的更广泛应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


