Microstructural characteristics and reaction mechanism of ammonium sulfate-promoted cuprite sulfidation
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
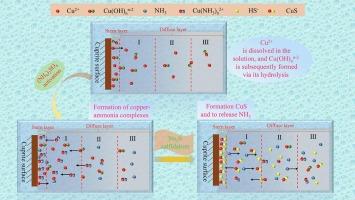
With the depletion of copper sulfide resources, efficient flotation technologies for low-grade copper oxides such as cuprite have garnered considerable attention. Due to the good hydrophobicity exhibited by copper sulfide, sulfidation is generally required for the flotation of cuprite. Meanwhile, ammonium sulfide ((NH4)2SO4) has been widely used as an activator in the sulfidation flotation of cuprite. However, the mechanism through which (NH4)2SO4 enhances the sulfidation effect remains to be systematically elucidated. The study of cuprite’s flotation behavior and surface wettability in this paper reveals that (NH4)2SO4 reduces the depressant effect of excessive Na2S on cuprite and enhances its flotation efficiency. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) analysis of sulfidation product ions (S2−, S− and Cu2S+) verified the (NH4)2SO4 enhancement of cuprite sulfidation. Microscopic morphology analysis indicates that cuprite surface roughness increased and sulfide products exhibited higher crystallinity and quantity following (NH4)2SO4-enhanced sulfidation. It was revealed by X-ray photoelectron spectroscopy (XPS) analyses that the sulfidation of cuprite was promoted by (NH4)2SO4, as demonstrated by the increase of hydrophobic S22− and Sn2− species on the cuprite surface; oxygen was also found to play a crucial role in this process. The main product of direct sulfidation was identified as Cu7.2S4 by Grazing-Incidence X-ray Diffraction (GIXRD), and the main product of (NH4)2SO4-enhanced sulfidation was also identified as Cu7.2S4 and Cu1.8S. Furthermore, it has been shown by studies on the solution components that [Cu(NH3)n]2+ are formed through the combination of (NH4)2SO4 with copper ions, thereby facilitating the dissolution of cuprite. Subsequently, [Cu(NH3)n]2+ and HS− are caused to react, resulting in the generation of copper sulfide and the release of NH3. A significant enhancement in cuprite’s sulfidation, a strengthened adsorption of NaBX, and an improved flotation efficiency are all achieved through the oxygen-ammonium synergistic effect.
硫酸铵促进铜硫化的微观结构特征及反应机理
随着硫化铜资源的日益枯竭,低品位氧化铜(如铜矿)的高效浮选技术已引起人们的广泛关注。由于硫化铜具有良好的疏水性,浮选铜一般需要进行硫化处理。同时,硫化铵((NH4)2SO4)作为活化剂已广泛应用于铜的硫化浮选。然而,(NH4)2SO4增强硫化效果的机理还有待系统阐明。本文对铜的浮选行为和表面润湿性进行了研究,发现(NH4)2SO4降低了过量Na2S对铜的抑制作用,提高了铜的浮选效率。飞行时间二次离子质谱(TOF-SIMS)分析了硫化产物离子(S2−、S−和Cu2S+),验证了(NH4)2SO4对铜硫化的增强作用。微观形貌分析表明,(NH4) 2so4强化硫化后,铜的表面粗糙度增加,硫化产物的结晶度和数量增加。x射线光电子能谱(XPS)分析表明,(NH4)2SO4促进了铜的硫化,铜表面的疏水性S22−和Sn2−物质增多;氧气也被发现在这个过程中起着至关重要的作用。经掠射x射线衍射(GIXRD)鉴定,直接硫化的主要产物为Cu7.2S4; (NH4) 2so4强化硫化的主要产物为Cu7.2S4和Cu1.8S。此外,对溶液组分的研究表明,[Cu(NH3)n]2+是由(NH4)2SO4与铜离子结合形成的,有利于铜的溶解。随后,[Cu(NH3)n]2+与HS−发生反应,生成硫化铜并释放NH3。氧铵协同作用显著增强了铜矿的硫化,增强了对NaBX的吸附,提高了浮选效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


