A ratiometric theranostic nanoplatform with acid-triggered drug release and glutathione-activated fluorescence turn-on
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Theragnostic nanomedicines that precisely coordinate drug delivery with imaging remain a significant challenge. Here, we report a novel nanoformulation that integrates acid-sensitive drug release with a glutathione (GSH)-activated fluorescence turn-on mechanism for targeted cancer therapy and real-time tracking. Our system is based on folic acid-functionalized zinc oxide quantum dots (QFZnO) loaded with a third-generation Casiopeina drug, IIIGCas, a copper-based coordination compound. Upon reaching the acidic tumor microenvironment, IIIGCas is released, subsequent intracellular GSH reduction converts the non-fluorescent Cu(II) complex into a highly fluorescent Cu(I) adduct, activating a distinct “turn-ON” signal. Computational studies reveal that this redox switch suppresses photoinduced electron transfer, restoring the emission of the drug's intrinsic curcumin-derived fluorophore and amplifying its fluorescence by sixfold. This mechanism creates a dual-emission system, enabling the simultaneous ratiometric tracking of nanocarrier (QFZnO) localization and drug activation. The nanoplatform demonstrated enhanced potency, showing statistically significant cytotoxicity in cervical and triple-negative breast cancer cell lines at far lower doses than free IIIGCas. In vivo, using zebrafish xenograft models, it achieved precise tumor targeting and a 90 % reduction in primary tumor area, while effectively illuminating secondary micrometastases. This work provides a generalizable blueprint for designing intelligent metal-complex delivery systems that optically report their own therapeutic activation in real-time.
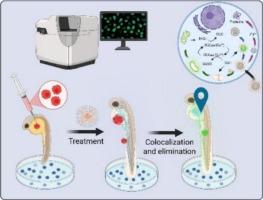
一种酸触发药物释放和谷胱甘肽激活荧光开启的比例治疗纳米平台
精确协调药物传递与成像的治疗诊断纳米药物仍然是一个重大挑战。在这里,我们报道了一种新的纳米制剂,该制剂将酸敏感药物释放与谷胱甘肽(GSH)激活的荧光开启机制结合起来,用于靶向癌症治疗和实时跟踪。我们的系统基于叶酸功能化氧化锌量子点(QFZnO),负载第三代Casiopeina药物IIIGCas,一种铜基配位化合物。在到达酸性肿瘤微环境后,IIIGCas被释放,随后细胞内GSH还原将非荧光Cu(II)复合物转化为高荧光Cu(I)加合物,激活一个独特的“打开”信号。计算研究表明,这种氧化还原开关抑制了光诱导的电子转移,恢复了药物固有的姜黄素衍生荧光团的发射,并将其荧光放大了六倍。该机制创建了一个双发射系统,实现了纳米载体(QFZnO)定位和药物激活的同时比率跟踪。纳米平台显示出增强的效力,在远低于游离IIIGCas剂量的情况下,对宫颈癌和三阴性乳腺癌细胞系显示出统计学上显著的细胞毒性。在体内,使用斑马鱼异种移植模型,它实现了精确的肿瘤靶向,原发肿瘤面积减少了90% %,同时有效地照亮了继发性微转移。这项工作为设计智能金属复合物递送系统提供了一个可概括的蓝图,该系统可以光学地实时报告其自身的治疗激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


