Electron transfer and hydrogen spillover Co-driven to enhance hydrogen evolution reaction over oxygen-deficient tungsten oxide-supported iridium clusters
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The development of low-loading precious metal-based electrocatalysts for hydrogen evolution reaction (HER) remains a research priority and technical challenge, requiring the effective utilization of robust interfacial interactions and synergistic effects between metal clusters and corresponding supporting substrates. Herein, a highly active electrocatalyst for hydrogen evolution with iridium (Ir) clusters boosted on oxygen-deficient tungsten oxide (W18O49) support is proposed via a one-pot hydrothermal strategy. The 1.16 wt% Ir/W18O49 catalyst demonstrates a low overpotential of 33 mV at 10 mA cm−2, along with superior Ir mass activity (13.9 A mg−1) at − 50 mV versus reversible hydrogen electrode (vs. RHE) that surpasses Ir/C, commercial 20 wt% platinum on carbon (Pt/C), and most reported precious metal-based catalysts. Theoretical calculations reveal that the synergistic modulation of electron transfer from Ir clusters to the W18O49 support and the hydrogen spillover effect regulate the hydrogen adsorption and desorption, thus enhancing the HER performance.
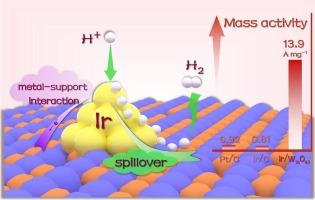
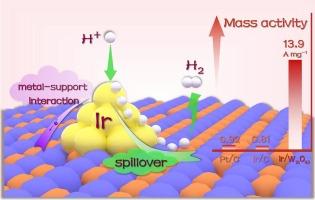
电子转移和氢溢出共同驱动在缺氧氧化钨负载的铱团簇上增强析氢反应
开发用于析氢反应的低负载贵金属电催化剂仍然是一个研究重点和技术挑战,这需要有效利用金属团簇和相应载体之间强大的界面相互作用和协同效应。本文提出了一种以缺氧氧化钨(W18O49)为载体,以铱(Ir)团簇为载体的高活性析氢电催化剂。1.16 wt% Ir/W18O49催化剂在10 mA cm - 2时具有33 mV的低过电位,在 − 50 mV时具有优于可逆氢电极(vs. RHE)的Ir质量活性(13.9 a mg - 1),超过Ir/C,商业20 wt%铂在碳上(Pt/C),以及大多数报道的贵金属基催化剂。理论计算表明,Ir簇向W18O49载体的电子转移和氢溢出效应协同调节了氢的吸附和解吸,从而提高了HER的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


