Breaking symmetry: enhanced propane dehydrogenation via asymmetric oxygen vacancies from vanadium doped ZrO2 catalysts
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Zirconia, ZrO2, has attracted considerable attention due to its outstanding catalytic performance in the propane dehydrogenation (PDH) reaction, primarily attributed to the high reactivity of coordinatively unsaturated zirconium sites (Zrcus) around oxygen vacancies. As an effective strategy for modulating the active sites on the zirconia surface, metal doping has been found to significantly optimize its catalytic performance. In current work, DFT calculations and microkinetic simulations were combined to systematically investigate the catalytic mechanism of vanadium-doped ZrO2 in the PDH reaction. V doping caused the significant changes of both geometry and electronic structure regarding of Zrcus, and in particularly V doping induced the formation of an asymmetric oxygen vacancy, where the adjacent V and Zrcus sites exhibit significant asymmetric electron distribution, thereby effectively enhancing catalytic activity. Specifically, V doping not only significantly reduces the energy barrier for C–H bond activation in propane but also facilitates hydrogen molecule desorption. Moreover, the reaction mechanism is also altered by V doping compared with pristine catalyst as the strong rate dependence on the product desorption observed on undoped surface is largely reduced after V doping which is benefit for product formation. This study provides important guidance for designing highly efficient and environmentally friendly PDH catalysts.
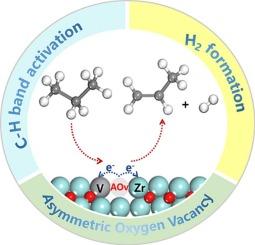
打破对称:通过不对称氧空位从钒掺杂的ZrO2催化剂中增强丙烷脱氢
氧化锆(ZrO2)因其在丙烷脱氢(PDH)反应中的优异催化性能而受到广泛关注,这主要是由于氧空位周围的配位不饱和锆(Zrcus)具有很高的反应活性。作为调节氧化锆表面活性位点的一种有效策略,金属掺杂可以显著优化氧化锆的催化性能。本研究将DFT计算和微动力学模拟相结合,系统地研究了钒掺杂ZrO2在PDH反应中的催化机理。V的掺杂使Zrcus的几何结构和电子结构发生了明显的变化,特别是V的掺杂引起了不对称氧空位的形成,其中相邻的V和Zrcus位点表现出明显的不对称电子分布,从而有效地提高了催化活性。具体来说,V掺杂不仅显著降低了丙烷中C-H键激活的能垒,而且有利于氢分子的脱附。此外,与原始催化剂相比,V掺杂还改变了反应机理,因为在未掺杂的表面上观察到的对产物脱附的强速率依赖性在V掺杂后大大降低,有利于产物的形成。该研究对设计高效、环保的PDH催化剂具有重要的指导意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


