Mechanisms of Hydroxylating Species Production by Dissolved Organic Matter Model Photosensitizers
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
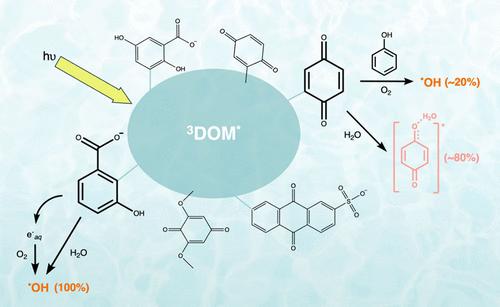
Hydroxyl radicals (•OH) are a species of interest in the environmental fate of contaminants due to their fast and nonselective reactions with both organic and inorganic compounds. Sources of •OH in surface waters include nitrate and nitrite photolysis, the photo-Fenton reaction, and dissolved organic matter (DOM) photolysis. The production mechanisms of •OH by DOM are classified based on their involvement of hydrogen peroxide (H2O2), known as the H2O2-dependent and H2O2-independent pathways. While the generation of •OH through the H2O2-dependent has been well-studied, the H2O2-independent pathway has remained unclear due to reactions between •OH (or other lower-energy hydroxylating species) and probe and quencher compounds. In this work, the pathways of •OH formation by six model quinones and hydroxybenzoic acids were investigated using methane and catalase to quench •OH and H2O2, respectively. This work suggests that quinones primarily generate lower-energy hydroxylating species but may form •OH in the presence of an electron donor through the H2O2-dependent pathway. Hydroxybenzoic acids were shown to produce free •OH through both H2O2-dependent and -independent pathways. Based on these results, we estimate that only 10–20% of all hydroxylating species produced by DOM in natural surface waters are free •OH, implying that previous work has overestimated •OH steady-state concentrations and their contribution to contaminant fate.
溶解有机物模型光敏剂产生羟基化物质的机制
羟基自由基(•OH)由于其与有机和无机化合物的快速和非选择性反应而在污染物的环境命运中引起了人们的兴趣。•OH在地表水中的来源包括硝酸盐和亚硝酸盐光解、光- fenton反应和溶解有机物(DOM)光解。DOM生成•OH的机制可根据其对过氧化氢(H2O2)的参与程度进行分类,分为H2O2依赖性途径和H2O2非依赖性途径。虽然通过h2o2依赖性生成•OH已经得到了很好的研究,但由于•OH(或其他低能羟基化物质)与探针和猝灭剂化合物之间的反应,不依赖h2o2的途径仍不清楚。本文研究了六种模式醌和对羟基苯甲酸分别用甲烷和过氧化氢酶淬灭•OH和H2O2生成•OH的途径。这项工作表明,醌主要产生低能羟基化物质,但可能在电子供体存在下通过h2o2依赖途径形成•OH。羟基苯甲酸通过h2o2依赖性和非依赖性途径产生游离•OH。基于这些结果,我们估计天然地表水中DOM产生的所有羟基化物质中只有10-20%是游离•OH,这意味着以前的工作高估了•OH稳态浓度及其对污染物命运的贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


