Sequential oxidation-reduction process for active chlorine distribution regulation on ammonia oxidation in the flow-through electrode system
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
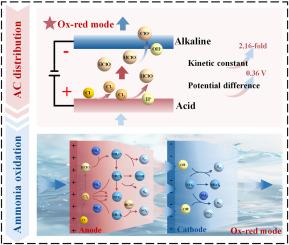
Chlorine-mediated flow-through electrode systems (Cl-FES) are considered a promising method for water treatment. However, the transformation and distribution of various reactive species in the Cl-FES remain unclear. In this study, the active chlorine (AC) distribution and electro-oxidation performance of oxidation-reduction (Ox-red) mode (anode-cathode) were systematically investigated. The pH distribution and linear sweep voltammetry curve revealed that the acid-to-alkaline transition zone in the Ox-red mode is conducive to suppressing the side reaction of oxygen evolution. The Ox-red mode achieved a 2.16-fold enhancement in AC generation rate compared to the reduction-oxidation (Red-ox) mode (cathode-anode). Meanwhile, SHapley Additive exPlanation (SHAP) analysis confirmed that flow rate was the predominant electrolytic parameter in the AC generation, with an optimal output of 92.31 mg/L achieved at 1.5 mL/min. The AC distribution at different flow rates indicated that an increased flow rate promotes the formation of hypochlorous acid (HClO) in the vicinity of the anode and inhibits the formation of chlorates. Furthermore, the ammonia electro-oxidation results demonstrated that the Ox-red mode exhibited a 2.38-fold improvement in ammonia removal rate and a high nitrogen selectivity of 94.51 %. Additionally, the chloramine byproducts generated in the interelectrode region decreased by 98.33 % after passing the cathode region. The overall findings offer insights into reactive species and byproducts regulation for effective and environmental-friendly electro-oxidation applications in flow-through electrode systems.
序贯氧化-还原过程中活性氯的分布调控对氨氧化的影响
氯介导的流动电极系统(Cl-FES)被认为是一种很有前途的水处理方法。然而,各种活性物质在Cl-FES中的转化和分布尚不清楚。本研究系统地研究了氧化-还原(Ox-red)模式(阳极-阴极)的活性氯(AC)分布和电氧化性能。pH分布和线性扫描伏安曲线表明,氧化-红模式下的酸碱过渡区有利于抑制析氧副反应。与还原-氧化(Red-ox)模式(阴极-阳极)相比,Ox-red模式的交流产生率提高了2.16倍。同时,SHapley Additive exPlanation (SHAP)分析证实,流速是AC代的主要电解参数,在1.5 mL/min的条件下,最佳产量为92.31 mg/L。不同流速下的AC分布表明,流速的增加促进了阳极附近次氯酸(HClO)的生成,抑制了氯酸盐的生成。此外,氨电氧化结果表明,氧化-红模式的氨去除率提高了2.38倍,氮选择性高达94.51%。通过阴极区后,电极间区氯胺副产物的生成减少了98.33%。总体研究结果为有效和环境友好型电氧化在直通电极系统中的应用提供了对反应物质和副产物调节的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


