Atomic-scale study on nanoparticle removal mechanism during post-Cu CMP cleaning process using ReaxFF MD
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The removal of silica (SiO2) nanoparticles on the surface of the copper (Cu) wafer during the post chemical mechanical polishing process has not yet been researched at the atomic level, making it difficult to achieve nanoparticle-free cleaning performance. In this work, the deposition and removal processes of SiO2 nanoparticles on Cu (1 1 1) surface in water, ammonium hydroxide and citric acid were studied by reactive force field molecular dynamics (ReaxFF-MD) simulation. The surface state of the copper substrate after pretreatment, the chemical bond evolution, and the mechanisms of different chemicals on nanoparticle removal were researched. The results show that water molecules promote the cleavage of Si-O-Cu bonds through chemical decomposition. Ammonium hydroxide dehydrogenates water molecules adsorbed on the copper surfaces to form hydroxyl groups through dissociation reaction, thereby increasing the electrostatic repulsion and suppressing the deposition. Simultaneously, more surface damage is produced since it could enhance the reactivity of Cu atoms. Citric acid could chemically adsorb onto the Cu substrate, hindering the contact between nanoparticles and the copper surface and promoting the lateral movement of nanoparticles. This work provides atomistic insights into the wafer cleaning process after chemical mechanical polishing, which is of great significance for the design of cleaning solutions.
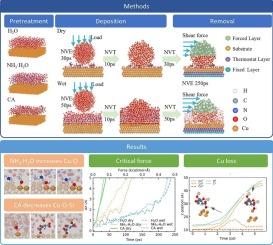
ReaxFF MD在cu - CMP后清洗过程中纳米颗粒去除机理的原子尺度研究
化学后机械抛光过程中对铜(Cu)晶片表面二氧化硅(SiO2)纳米颗粒的去除尚未在原子水平上进行研究,难以实现无纳米颗粒的清洁性能。本文采用反应力场分子动力学(ReaxFF-MD)模拟研究了SiO2纳米颗粒在水、氢氧化铵和柠檬酸中Cu(11 11)表面的沉积和去除过程。研究了预处理后铜基体的表面状态、化学键的演化以及不同化学物质去除纳米颗粒的机理。结果表明,水分子通过化学分解促进Si-O-Cu键的断裂。氢氧化铵通过解离反应使吸附在铜表面的水分子脱氢,形成羟基,从而增加静电斥力,抑制沉积。同时,由于铜原子的反应性增强,会产生更多的表面损伤。柠檬酸可以化学吸附在Cu衬底上,阻碍纳米颗粒与铜表面的接触,促进纳米颗粒的横向运动。本研究为化学机械抛光后的晶圆清洗过程提供了原子的视角,对清洗溶液的设计具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


