Growth progression driven by c-di-GMP in photogranules under hydrostatic conditions: polysaccharides biosynthesis and microbial interactions
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Hydrostatically formed photogranules (HSPs) are spontaneously formed algal-bacterial aggregates, wherein sticky polysaccharides (PS) intertwine with the microbial moiety to form cohesive structures. However, the mechanism of algal-bacterial self-immobilization for spherical granules remains unclear. Herein, we elucidated cyanobacterial-bacterial interactions by applying multi-omics analysis to reveal the self-aggregation mechanism of HSPs. Under optimal conditions, mature HSPs were formed within 30 days with the success rate of cultivation exceeding 90 %. During the phototrophic bloom and granulation phases, PS secretion was significantly correlated with the levels of c-di-GMP (p < 0.01). Notably, alginate (24.43 % ± 4.23 %) was the most abundant metabolite among the PS compositions, and the cross-feeding between filamentous cyanobacteria (FC) and symbiotic bacteria for alginate constituted the core of intercellular relationships in HSPs. FC secreted c-di-GMP via a Wsp chemosensory-like system and promoted the alginate production. Acidobacteriota-affiliated bacteria exhibited expression of gene algl (4.47 %) to utilize alginate, contributing to PS polymerization and a tight assembly of FC and bacteria. In return, bacteria provided growth factors and facilitated public-goods (vitamins and cofactors) exchanges with FC. Overall, the metabolic characterizations advanced the photogranulation mechanism from the aspect of cross-feeding mediated by c-di-GMP, highlighting the significant role of PS in the growth progressions of HSPs.
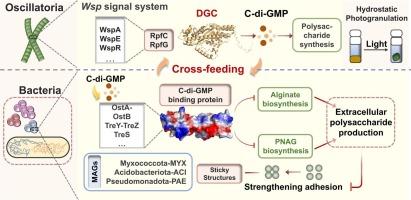
静水条件下c-二gmp驱动的光颗粒生长过程:多糖生物合成和微生物相互作用
流体静力形成的光颗粒(HSPs)是自发形成的藻-细菌聚集体,其中粘性多糖(PS)与微生物部分相互缠绕形成内聚结构。然而,藻-细菌自固定球形颗粒的机制尚不清楚。在此,我们通过多组学分析阐明了蓝藻与细菌的相互作用,揭示了热敏感蛋白的自聚集机制。在最佳条件下,30 d内形成成熟热蛋白,培养成功率超过90% %。在光华期和肉芽期,PS分泌量与c-二- gmp水平呈极显著相关(p <; 0.01)。值得注意的是,藻酸盐(24.43 % ± 4.23 %)是PS组成中最丰富的代谢物,丝状蓝藻(FC)与共生细菌对藻酸盐的交叉取食构成了HSPs细胞间关系的核心。FC通过Wsp化学感觉系统分泌c-二gmp,促进海藻酸盐的产生。酸杆菌属细菌表达了algl基因(4.47 %)来利用海藻酸盐,促进PS聚合和FC与细菌的紧密组装。作为回报,细菌提供了生长因子,并促进了与FC的公共产品(维生素和辅助因子)交换。综上所述,这些代谢特征从c-di-GMP介导的交叉摄食的角度推进了光造粒机制,突出了PS在HSPs生长过程中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


