Progress on electrochemical preparation of inorganic materials in deep eutectic solvents-mediated organic electrolytes
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
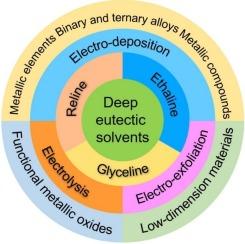
Excellent electrical conductivity, wide potential windows, high solubility, and low vapor pressure are typical of deep eutectic solvents, which endow these newly emerging solvents with significant advantages in electrochemical preparation. Deep eutectic solvents have proven effective for the electrochemical preparation of inorganic materials, either as complete electrolytes or as conductive mediators in electrolyte systems. The related technical route comprises electrodeposition, electro-exfoliation, and electrolytic redox, and the resultant materials have demonstrated impressive functional enhancements. This review begins with a concise overview of compositions, characteristics, and applications of the deep eutectic solvents. Whereafter, progress in electrochemically preparing inorganic materials in deep eutectic solvents-mediated organic electrolytes is retrospectively emphasized. The deep eutectic solvents discussed are limited to those derived from choline quaternary ammonium salts, especially Reline, Ethaline, and Glyceline. The synthesis includes electrodeposition to synthesize elementary substances, bimetallic alloys, trimetallic alloys, and metal-nonmetal compounds. The electrochemical process involves electro-exfoliation to produce two-dimensional materials, exemplified by graphene. The preparation also covers the electrolysis to prepare metallic oxides. Finally, the electrolytic preparation of functional inorganic materials in deep eutectic solvent-mediated organic electrolytes, based on two-electrode systems, is discussed and envisioned.
深共晶溶剂-有机电解质中无机材料的电化学制备研究进展
深共晶溶剂具有优良的导电性、宽的电位窗、高的溶解度和低的蒸气压等特点,在电化学制备中具有显著的优势。深共晶溶剂已被证明对无机材料的电化学制备是有效的,无论是作为完整的电解质还是作为电解质系统中的导电介质。相关的技术路线包括电沉积、电剥离和电解氧化还原,所得材料已显示出令人印象深刻的功能增强。本文首先简要介绍了深共晶溶剂的组成、特点和应用。然后,回顾了在深共晶溶剂介导的有机电解质中电化学制备无机材料的进展。所讨论的深共晶溶剂仅限于来自胆碱季铵盐的溶剂,特别是Reline、Ethaline和Glyceline。合成包括电沉积合成基本物质、双金属合金、三金属合金和金属-非金属化合物。电化学过程包括电剥离以产生二维材料,例如石墨烯。制备还包括电解制备金属氧化物。最后,对基于双电极体系的深度共晶溶剂介导有机电解质中功能无机材料的电解制备进行了讨论和展望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


