Simultaneous Ca/Li separation and Ca-based layered double hydroxide synthesis from oilfield brine for heavy metal removal in electroplating wastewater
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Oilfield brine, a by-product of oil and gas extraction, contains valuable resources such as Li+, K+ and Ca2+, offering potential utilisation benefits but also presenting separation challenges. We developed a reaction-coupled separation strategy to separate Ca2+ and Li+ while simultaneously synthesising a calcium-based layered double hydroxide (Ca-based LDH) from the brine. This technology enables the recovery of over 90 % of Ca2+ into the solid product, with 98.5 % of Li+ retained in the liquid phase, achieving highly efficient separation of Ca2+ and Li+. The resulting Ca-based LDH demonstrated excellent capacity for removing heavy metals, such as Cu2+, Ni2+ and Zn2+, from electroplating wastewater. The removal capacities reached up to 384.2 mg/g for Cu2+, 319.3 mg/g for Ni2+ and 367.9 mg/g for Zn2+. In both single-cation and mixed-cation wastewaters, metal concentrations were dramatically reduced to parts-per-billion (ppb) levels. Kinetic studies indicated rapid adsorption behaviour of Cu2+, Ni2+ and Zn2+ by the Ca-based LDH. Mechanistic analysis revealed that Ca2+ ions in the LDH layers are isomorphously substituted by Cu2+, Ni2+ and Zn2+ from the solution, forming Cu-, Ni- and Zn-containing LDHs with enhanced structural stability. Additionally, the released Ca2+ ions precipitate with CO₃2−, derived either from intercalated anions or carbonate in the water. Such conditions promote further substitution of Ca2+ in the LDH layers by Cu2+, Ni2+ and Zn2+, resulting in robust metal removal and ultralow residue concentrations in the treated wastewater. This work presents an efficient, economical and sustainable approach for the simultaneous separation and utilisation of oilfield brine resources and treatment of electroplating wastewater.
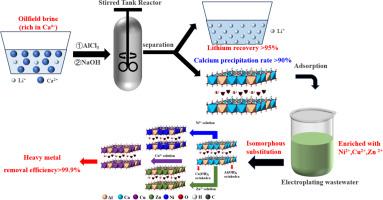
油田卤水Ca/Li同时分离及Ca基层状双氢氧化物合成去除电镀废水中重金属
油田卤水是石油和天然气开采的副产品,含有宝贵的资源,如Li+、K+和Ca2+,具有潜在的利用效益,但也存在分离挑战。我们开发了一种反应耦合分离策略来分离Ca2+和Li+,同时从盐水中合成钙基层状双氢氧化物(Ca-based LDH)。该技术使固相产物中Ca2+的回收率超过90%,液相中Li+的回收率为98.5%,实现了Ca2+和Li+的高效分离。结果表明,ca基LDH对电镀废水中的重金属Cu2+、Ni2+和Zn2+具有良好的去除能力。对Cu2+、Ni2+和Zn2+的去除率分别达到384.2 mg/g、319.3 mg/g和367.9 mg/g。在单阳离子和混合阳离子废水中,金属浓度都大大降低到十亿分之一(ppb)的水平。动力学研究表明,ca基LDH对Cu2+、Ni2+和Zn2+具有快速吸附行为。机制分析表明,LDH层中的Ca2+离子被溶液中的Cu2+、Ni2+和Zn2+同构取代,形成含Cu、Ni和zn的LDH,结构稳定性增强。此外,释放的Ca2+离子与CO₃2−沉淀,它们要么来自插层阴离子,要么来自水中的碳酸盐。这样的条件促进了LDH层中的Ca2+进一步被Cu2+、Ni2+和Zn2+取代,从而实现了强有力的金属去除和处理废水中的超低残留浓度。为油田卤水资源的分离利用和电镀废水的处理提供了一种高效、经济、可持续的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


