Revealing the mechanism of singlet oxygen formation during the activation of Peroxymonosulfate by Feco dual-atom catalysts
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
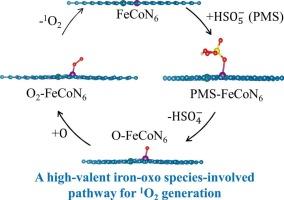
The development of selective reactive oxygen species is crucial for the efficient removal of organic contaminants. Iron‑cobalt dual-atom catalysts (FeCoDACs) show great promise for generating singlet oxygen (1O2), a highly selective species, through the activation of peroxymonosulfate (PMS), but the formation mechanism remains unclear. In this study, FeCoDACs were synthesized via a facile pyrolysis method, and the activation of PMS by these catalysts for pollutant degradation and 1O2 generation was systematically investigated. Near-complete degradation of sulfamethoxazole was achieved after 30 min of reaction in the presence of 0.5 mM PMS and 0.05 g/L FeCoDACs. The formation of 1O2 during PMS activation was confirmed by electron paramagnetic resonance spectroscopy, and the formation kinetics were examined through quantification of 2,2,6,6-tetramethylpiperidine 1-oxide. Due to the high selectivity of 1O2, the presence of common water constituents—except for natural organic matter—did not adversely affect the treatment performance. For the same reason, the oxidative system performed well in treating real water matrices. Density functional theory calculations revealed that cleavage of the S O bond in PMS, rather than the peroxone bond occurred during activation by FeCoDACs, leading to the formation of high-valent iron-oxo species as a key intermediate for 1O2. The oxygen in the iron-oxo species then combined with oxygen from an adjacent site to form an OO* intermediate, which dissociated from the catalyst surface, resulting in the formation of 1O2. These findings are expected to enhance understanding of 1O2 formation during PMS activation by DACs
O bond in PMS, rather than the peroxone bond occurred during activation by FeCoDACs, leading to the formation of high-valent iron-oxo species as a key intermediate for 1O2. The oxygen in the iron-oxo species then combined with oxygen from an adjacent site to form an OO* intermediate, which dissociated from the catalyst surface, resulting in the formation of 1O2. These findings are expected to enhance understanding of 1O2 formation during PMS activation by DACs
揭示了Feco双原子催化剂活化过氧单硫酸盐过程中单线态氧的形成机理
选择性活性氧的开发是有效去除有机污染物的关键。铁-钴双原子催化剂(FeCoDACs)通过活化过氧单硫酸盐(PMS)产生单线态氧(1O2),这是一种高度选择性的物质,但其形成机制尚不清楚。本研究采用易热解法合成了FeCoDACs,并系统研究了这些催化剂对PMS降解污染物和生成1O2的活化作用。在0.5 mM PMS和0.05 g/L FeCoDACs的存在下,反应30 min后,磺胺甲异唑几乎完全降解。通过电子顺磁共振波谱法证实了PMS活化过程中1O2的生成,并通过2,2,6,6-四甲基哌啶1-氧化物的定量分析了生成动力学。由于1O2的高选择性,除了天然有机物外,常见水组分的存在对处理性能没有不利影响。由于同样的原因,氧化系统在处理实际水基质方面表现良好。密度泛函理论计算表明,在FeCoDACs的激活过程中,PMS中的SO键而不是过氧酮键发生了裂解,从而形成了作为1O2关键中间体的高价氧化铁。然后,铁氧中的氧与邻近位点的氧结合,形成OO*中间体,从催化剂表面解离,形成1O2。这些发现有望加深对DACs激活PMS过程中1O2形成的理解
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


