Hypoxia promotes airway differentiation in the human lung epithelium
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Human lungs experience dynamic oxygen tension during development. Here, we show that hypoxia directly regulates human lung epithelial cell identity using tissue-derived organoids. Fetal multipotent lung epithelial progenitors remain undifferentiated in a self-renewing culture condition under normoxia but spontaneously differentiate toward multiple airway cell types and inhibit alveolar differentiation under hypoxia. Using chemical and genetic tools, we demonstrate that hypoxia-induced airway differentiation depends on hypoxia-inducible factor (HIF) activity, with HIF1α and HIF2α differentially regulating progenitor fate decisions. KLF4 and KLF5 are direct HIF targets that promote basal and secretory cell fates. Moreover, hypoxia is sufficient to convert alveolar type 2 cells derived from both human fetal and adult lungs to airway cells, including aberrant basal-like cells that exist in human fibrotic lungs. These findings reveal roles for hypoxia and HIF activity in the developing human lung epithelium and have implications for aberrant cell fate changes in pathological lungs.
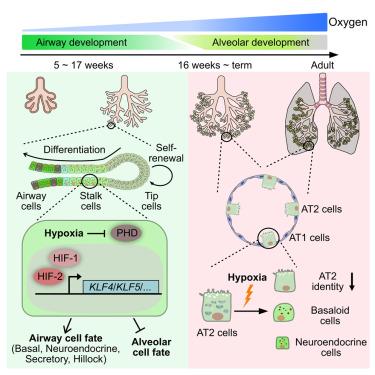
缺氧促进人肺上皮的气道分化
人的肺在发育过程中经历动态氧张力。在这里,我们表明缺氧直接调节人肺上皮细胞身份使用组织来源的类器官。胚胎多能性肺上皮祖细胞在常氧条件下的自我更新培养条件下保持未分化,但在缺氧条件下自发分化为多种气道细胞类型并抑制肺泡分化。利用化学和遗传学工具,我们证明缺氧诱导的气道分化取决于缺氧诱导因子(HIF)活性,HIF1α和HIF2α对祖细胞命运的决定有不同的调节作用。KLF4和KLF5是HIF的直接靶点,可促进基础细胞和分泌细胞的命运。此外,缺氧足以将来自人胎儿和成人肺的肺泡2型细胞转化为气道细胞,包括存在于人纤维化肺中的异常基底样细胞。这些发现揭示了缺氧和HIF活性在发育中的人肺上皮中的作用,并对病理肺中异常细胞命运的变化具有影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


