Recombinant glutamine synthetase originating from high ammonium-assimilating Bacillus subtilis effectively reduces NH3 emissions and enhances the conversion into amino acids in liquid manure
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Excessive ammonia (NH3) emissions from livestock manure contribute to environmental pollution and nitrogen (N) loss, yet effective mitigation strategies remain limited. In this study, Bacillus strains capable of utilizing ammonia nitrogen (NH4+) were screened from 166 probiotic strains. Nine strains exhibited utilization rates above 65.0 %, with B. subtilis M246 exceeding 80.0 %. Genome sequencing revealed the absence of key nitrification genes (e.g., amoCAB), suggesting NH4+ is metabolized primarily through assimilation via the GS/GOGAT pathway. The glnA gene, encoding glutamine synthetase (GS), was identified as central to this process but was transcriptionally repressed under high NH4+ concentrations (100 mM). To overcome this, glnA was heterologously expressed in Pichia pastoris, and the recombinant GS applied to liquid manure. GS treatments reduced NH3 emissions by 19.57 %–45.91 %, outperforming M246 (6.45 %), and significantly decreasing total nitrogen loss (18.53 %–24.30 %) compared to CK (32.71 %, p < 0.001) and M246 (29.31 %, p < 0.001 for 1 % and 5 % GS; p = 0.003 for 2 % GS). Moreover, GS reduced nitrous oxide emissions by 18.29 %–33.59 % relative to CK, and increased readily degradable proteins, promoting their conversion into humic substances and significantly elevating glutamine (p < 0.001) and glutamate (p = 0.001). GS significantly enriched microbial genera associated with N retention and mitigated emissions (p = 0.049); Acholeplasma and Sedimentibacter were enriched in both GS and M246 treatments, while Sphaerochaeta was specific to GS. Therefore, exogenous GS effectively reduces gaseous emissions, enhances N retention, promotes NH4+ conversion into amino acids, and improves the biofertilizer quality of liquid manure.
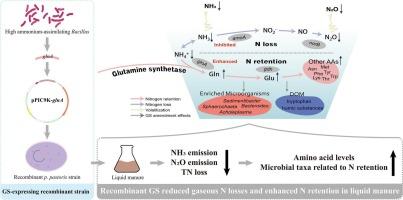
源自高氨同化性枯草芽孢杆菌的重组谷氨酰胺合成酶能有效减少氨的排放,提高粪便中氨基酸的转化
牲畜粪便中过量的氨(NH3)排放会造成环境污染和氮(N)损失,但有效的缓解战略仍然有限。本研究从166株益生菌中筛选出能利用氨氮(NH4+)的芽孢杆菌菌株。9株菌株的利用率在65.0 %以上,其中枯草芽孢杆菌M246的利用率在80.0 %以上。基因组测序显示缺少关键的硝化基因(如amoCAB),表明NH4+主要通过GS/GOGAT途径同化代谢。编码谷氨酰胺合成酶(GS)的glnA基因被认为是这一过程的核心,但在高NH4+浓度下转录受到抑制(100 mM)。为了克服这一问题,我们在毕赤酵母中异源表达glnA,并将重组GS应用于液体粪便中。GS治疗NH3排放减少了19.57 % % -45.91,优于M246(6.45 %),并显著降低总氮损失(18.53 % -24.30 %)比CK(32.71 % p & lt; 0.001)和M246(29.31 % p & lt; 0.001 1 % 5 % GS; p = 0.003 2 % GS)。此外,与对照相比,GS降低了18.29 % -33.59 %的氧化亚氮排放量,增加了易于降解的蛋白质,促进了它们向腐殖质物质的转化,并显著提高了谷氨酰胺(p <; 0.001)和谷氨酸(p = 0.001)。GS显著富集与N保持和减少排放相关的微生物属(p = 0.049);在GS和M246处理中均富集了石原体和沉积杆菌,而Sphaerochaeta对GS具有特异性。因此,外源GS有效地减少了气体排放,增强了N的保留,促进了NH4+向氨基酸的转化,提高了液肥的生物肥料品质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


