Ensuring quality control in HME, a quantitative analysis of hydrocortisone filament using a portable Raman spectrometer
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-09-19
DOI:10.1016/j.saa.2025.126968
引用次数: 0
Abstract
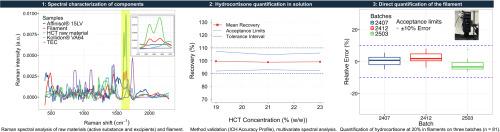
3D printing is a breakthrough in drug development, offering advantages like personalized medication and the ability to create complex drug formulations. Ensuring safety and efficacy of these printed medications requires rigorous quality control, for which Raman spectroscopy is a powerful tool. This technique can be integrated into the hot melt extrusion (HME) process or the printing process itself, analyzing the drug-content in filament after its production and before it is melted and formed into the final dosage form.
This project focused on developing a quantitative analytical method using a portable Raman spectrometer to measure the concentration of hydrocortisone (HCT) in a filament. This filament acts as a pharmaceutical ink, designed for printing solid oral forms for individualized dosing.
Following ICH guidelines, a validated method for HCT quantification in solution was established. This method was then successfully adapted for direct quantification of HCT within the filament composed of 20 % HCT and excipients. The initial step involved defining a specific spectral region unique to HCT. Preprocessing methods were optimized, including smoothing, baseline correction, derivatives and Extended Multiplicative Signal Correction, used to mitigate unwanted spectral variations.
The method proved highly accurate for the target HCT concentration across three filament batches, achieving a mean absolute error of 2.96 %. This project highlights the value of using a portable Raman probe to control the quality of the filament either at the output of HME or directly at the point of care, in order to verify the quality of the received filament.
为了保证HME的质量控制,使用便携式拉曼光谱仪对氢化可的松灯丝进行了定量分析。
3D打印是药物开发的一个突破,它提供了个性化治疗和创建复杂药物配方的能力等优势。确保这些打印药物的安全性和有效性需要严格的质量控制,拉曼光谱是一个强大的工具。该技术可集成到热熔挤压(HME)工艺或打印工艺本身,在长丝生产后和熔化形成最终剂型之前分析其药物含量。本项目的重点是开发一种使用便携式拉曼光谱仪测量灯丝中氢化可的松(HCT)浓度的定量分析方法。这种丝作为医药油墨,设计用于打印固体口服形式的个性化剂量。根据ICH指南,建立了溶液中HCT定量的有效方法。然后,该方法成功地适用于20% HCT和辅料组成的长丝内HCT的直接定量。最初的步骤包括定义HCT特有的特定光谱区域。优化了预处理方法,包括平滑、基线校正、导数和扩展乘法信号校正,用于减轻不必要的频谱变化。结果表明,该方法对目标HCT浓度具有较高的准确度,平均绝对误差为2.96%。该项目强调了使用便携式拉曼探针在HME输出或直接在护理点控制灯丝质量的价值,以验证接收灯丝的质量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


