Synthesis of new chlorin e6 derivatives bearing galactose moieties on the macrocyclic periphery and their structure–activity relationships
IF 3.7
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Journal of photochemistry and photobiology. B, Biology
Pub Date : 2025-09-16
DOI:10.1016/j.jphotobiol.2025.113268
引用次数: 0
Abstract
Recent studies of photosensitizers have increasingly focused on improving their aqueous solubility, reducing dark toxicity and enhancing photodynamic activity. In our work, we have synthesized and characterized a series of chlorin e6 derivatives varying in the number of galactose moieties in the molecule and way they are linked to the chlorin macrocycle. We established that the quantity and localization of galactose moieties in the macrocycle affects both dark and photoinduced cytotoxicity. All of the studied derivatives exhibited singlet oxygen quantum yield photogeneration comparable to that of the clinically approved chlorin e6 dimeglumine (Photoditazine®) with marginal exceeding. Although the incorporation of galactose moieties to the periphery of the chlorin macrocycle led to slightly decreased photostability of resulting derivatives, it did not significantly hinder their photodynamic activity. Moreover, cellular uptake of the obtained derivatives was significantly improved compared to that of Photoditazine®. Collectively, favorable spectral, photochemical, photobiological characteristics, along with improved aqueous solubility, suggest that chlorin e6 derivatives with peripheral galactose substituents are promising photosensitizers for clinical applications.
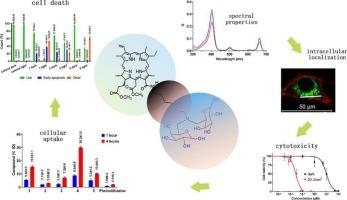
含半乳糖大环的氯代e6衍生物的合成及其构效关系。
近年来对光敏剂的研究越来越集中在改善其水溶性、降低暗毒性和光动力活性方面。在我们的工作中,我们合成并表征了一系列氯e6衍生物,这些衍生物在分子中半乳糖部分的数量和它们与氯大环的连接方式上都有所不同。我们确定了半乳糖部分在大环中的数量和定位影响暗和光诱导的细胞毒性。所有被研究的衍生物都表现出单线态氧量子产率,与临床批准的氯e6二甲基胺(photodiazine®)的产率相当,但边际超过。虽然半乳糖部分掺入到氯大环的外围会导致衍生物的光稳定性略有下降,但不会显著阻碍其光动力活性。此外,与Photoditazine®相比,所获得的衍生物的细胞摄取显着提高。总的来说,良好的光谱,光化学,光生物学特性,以及改善的水溶性,表明具有外周半乳糖取代基的氯e6衍生物是临床应用的有前途的光敏剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.10
自引率
1.90%
发文量
161
审稿时长
37 days
期刊介绍:
The Journal of Photochemistry and Photobiology B: Biology provides a forum for the publication of papers relating to the various aspects of photobiology, as well as a means for communication in this multidisciplinary field.
The scope includes:
- Bioluminescence
- Chronobiology
- DNA repair
- Environmental photobiology
- Nanotechnology in photobiology
- Photocarcinogenesis
- Photochemistry of biomolecules
- Photodynamic therapy
- Photomedicine
- Photomorphogenesis
- Photomovement
- Photoreception
- Photosensitization
- Photosynthesis
- Phototechnology
- Spectroscopy of biological systems
- UV and visible radiation effects and vision.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


