Unraveling the hnRNPK/miR-133a-3p/UCP2 axis: a novel regulatory circuit governing porcine skeletal muscle satellite cell fate
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Objective
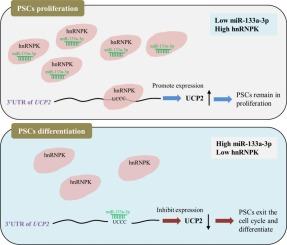
MicroRNAs (miRNAs) post-transcriptionally regulate gene expression by targeting mRNA 3’UTRs, critically influencing skeletal muscle development. While miR-133a-3p is implicated in myogenesis, its regulatory interplay with RNA-binding proteins (RBPs) in porcine skeletal muscle satellite cells (PSCs) remains unexplored. This study elucidates the functional role and molecular mechanism of miR-133a-3p through a novel hnRNPK/miR-133a-3p/UCP2 axis in PSCs.
Methods
RT-qPCR and western blot were used for gene expression analysis. miR-133a-3p mimic and miR-133a-3p inhibitor were conducted to overexpression and knockdown the expression of miR-133a-3p in PSCs, respectively. The hnRNPK overexpression vector was designed using the pcDNA3.1(+) vector. 5-ethynyl-2′-deoxyuridine (EdU) assays were conducted for cell proliferation. Immunofluorescence detection was employed for cell differentiation analysis. The dual-luciferase reporter assay, RNA immunoprecipitation (RIP) assay and biotin-labeled miRNA pull-down assays were utilized for regulatory mechanism analysis of hnRNPK/miR-133a-3p/ UCP2.
Results
Transcriptional profiling revealed miR-133a-3p upregulation during PSCs differentiation. Gain- and loss-of-function assays demonstrated that miR-133a-3p suppresses proliferation but promotes differentiation; its inhibition yielded opposite effects. Comprehensive mechanistic studies (dual-luciferase, RIP, and biotin pull-down assays) identified hnRNPK as a direct binding partner of miR-133a-3p. Subsequent validation confirmed UCP2 as a downstream target, with hnRNPK attenuating miR-133a-3p-mediated UCP2 repression. Notably, hnRNPK antagonized miR-133a-3p's pro-differentiation effects, revealing its role as a negative myogenesis regulator.
Conclusion
Collectively, we report a novel hnRNPK/miR-133a-3p/UCP2 axis governing mitochondrial gene expression and PSCs differentiation. This work advances understanding of RBP-miRNA interaction in post-transcriptional myogenic regulation and provides new targets for muscle biology and regenerative medicine.
揭示hnRNPK/miR-133a-3p/UCP2轴:调控猪骨骼肌卫星细胞命运的新调控回路
目的:MicroRNAs (miRNAs)通过靶向mRNA 3'UTRs转录后调控基因表达,对骨骼肌发育具有重要影响。虽然miR-133a-3p与肌肉发生有关,但其与猪骨骼肌卫星细胞(PSCs)中rna结合蛋白(rbp)的调节相互作用仍未被探索。本研究通过新的hnRNPK/miR-133a-3p/UCP2轴在psc中阐明了miR-133a-3p的功能作用和分子机制。方法:采用RT-qPCR和western blot技术进行基因表达分析。miR-133a-3p mimic和miR-133a-3p inhibitor分别在PSCs中过表达和敲低miR-133a-3p的表达。采用pcDNA3.1(+)载体设计hnRNPK过表达载体。5-乙基-2'-脱氧尿苷(EdU)检测细胞增殖。采用免疫荧光法进行细胞分化分析。采用双荧光素酶报告基因法、RNA免疫沉淀(RIP)法和生物素标记miRNA下拉法分析hnRNPK/miR-133a-3p/ UCP2的调控机制。结果:转录谱显示miR-133a-3p在psc分化过程中上调。功能获得和功能丧失实验表明,miR-133a-3p抑制增殖,但促进分化;它的抑制作用产生了相反的效果。综合机制研究(双荧光素酶、RIP和生物素下拉测定)确定hnRNPK是miR-133a-3p的直接结合伙伴。随后的验证证实UCP2是下游靶点,hnRNPK减弱了mir -133a-3p介导的UCP2抑制。值得注意的是,hnRNPK拮抗miR-133a-3p的促分化作用,揭示了其作为负向肌生成调节剂的作用。结论:总的来说,我们报告了一个新的hnRNPK/miR-133a-3p/UCP2轴控制线粒体基因表达和PSCs分化。这项工作促进了对RBP-miRNA在转录后肌生成调控中的相互作用的理解,并为肌肉生物学和再生医学提供了新的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


