LiCl-driven direct synthesis of mono-protected esters from long-chain dicarboxylic fatty acids
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A one-step mono-esterification method for long-chain dicarboxylic fatty acids [HO2C(CH2)nCO2H; n ≥ 14] was developed using TFAA (trifluoroacetic anhydride) and LiCl as esterification reagents. This approach was particularly effective for synthesizing mono tert-butyl esters, which are key intermediates in the production of segments of semaglutide and tirzepatide—two blockbuster drugs with their 2024 sales valued in billions of dollars. The addition of LiCl critically enhanced the monoester selectivity over diester formation. Mechanistic studies suggest that this selectivity originates from a shielding effect, where LiCl interacts with one terminal carboxylic acid group.
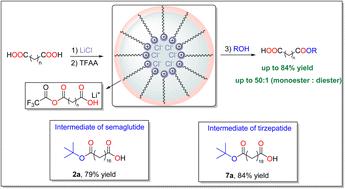
由长链二羧基脂肪酸直接合成单保护酯。
长链二羧酸一步单酯化法[HO2C(CH2) n CO2H]以TFAA(三氟乙酸酐)和LiCl为酯化试剂制备了n≥14]。这种方法在合成单叔丁基酯方面特别有效,而单叔丁基酯是生产西马鲁肽和替西帕肽片段的关键中间体,这两种重磅药物的销售额在2024年将达到数十亿美元。与二酯形成相比,LiCl的加入显著提高了单酯的选择性。机制研究表明,这种选择性源于屏蔽效应,其中LiCl与一个末端羧酸基团相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


