Arsenic trioxide enhances the inhibitory effect of lenvatinib on hepatocellular carcinoma through HMOX1-mediated ferroptosis
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Currently, Lenvatinib, a tyrosine kinase inhibitor, is used as a first-line treatment for advanced hepatocellular carcinoma (HCC), but its efficacy remains unsatisfactory. Therefore, we investigated the effect of Lenvatinib in combination with Arsenic trioxide (ATO) on HCC and the underlying mechanisms. The antitumor activity of the combination was evaluated in vitro using MTT and colony formation assays, and in vivo using xenograft models in nude mice. mRNA-seq was performed to explore the potential mechanisms. Reactive oxygen species (ROS) and intracellular Fe2+ were measured to evaluate ferroptosis in HCC cells. Our findings showed that the addition of ATO significantly enhanced the anti-HCC effects of Lenvatinib both in vitro and in vivo. Moreover, RNA sequencing analysis indicated that ATO augmented the anti-HCC effect of Lenvatinib by inducing ferroptosis. Both ATO alone and the combination treatment markedly induced a significant elevation of ROS and ferroptosis, which was effectively blocked by the administration of ferrostatin-1 and deferoxamine mesylate. Furthermore, ATO upregulated the levels of heme oxygenase 1 (HMOX1) and Fe2+ in HCCLM3 and Huh7 cells. Knockdown of HMOX1 attenuated the effect of ATO on HCC cell viability, ROS and Fe2+ levels in HCC cells. Additionally, ATO and the combination of Lenvatinib and ATO also decreased the expression of GPX4 protein both in vitro and in vivo. In conclusion, ATO enhances the antitumor activity of Lenvatinib against HCC by inducing ferroptosis through upregulation of HMOX1 and downregulation GPX4.
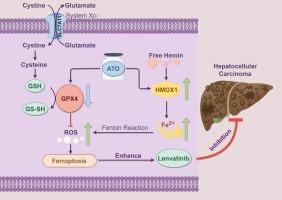
三氧化二砷通过hmox1介导的铁凋亡增强lenvatinib对肝癌的抑制作用
目前,酪氨酸激酶抑制剂Lenvatinib被用作晚期肝细胞癌(HCC)的一线治疗,但其疗效仍不理想。因此,我们研究Lenvatinib联合三氧化二砷(ATO)对HCC的影响及其潜在机制。体外用MTT法和菌落形成法评估联合用药的抗肿瘤活性,体内用裸鼠异种移植模型评估联合用药的抗肿瘤活性。通过mRNA-seq来探索潜在的机制。通过测定活性氧(ROS)和细胞内铁离子(Fe2+)来评估HCC细胞的铁下垂。我们的研究结果表明,ATO的加入显著增强了Lenvatinib在体外和体内的抗hcc作用。此外,RNA测序分析表明,ATO通过诱导铁下垂增强Lenvatinib的抗hcc作用。ATO单独和联合治疗均显著诱导ROS和铁下垂显著升高,这可通过给予他铁素-1和甲磺酸去铁胺有效阻断。此外,ATO上调HCCLM3和Huh7细胞中血红素加氧酶1 (HMOX1)和Fe2+的水平。敲低HMOX1可减弱ATO对HCC细胞活力、ROS和Fe2+水平的影响。此外,ATO以及Lenvatinib与ATO合用也降低了GPX4蛋白在体外和体内的表达。综上所述,ATO通过上调HMOX1和下调GPX4,诱导Lenvatinib对HCC的抗肿瘤活性增强。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


