Formation mechanisms of chlorophenylacetonitriles from aromatic amino acids in chloramine disinfection
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Chlorophenylacetonitriles (CPANs), a class of nitrogenous disinfection byproducts (DBPs) with high cytotoxicity, are increasingly detected in chloraminated water systems. However, their formation mechanisms from aromatic amino acids (ArAAs), critical nitrogen-rich precursors in proteinaceous waters, remain poorly understood. This study systematically investigated the intermediates, reactive sites, nitrogen sources, and pathways involved in CPANs formation from phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp) under chloramine disinfection. A total of 26 DBPs were identified, including 7 CPANs, with Trp exhibiting the highest diversity (18 species). 15N isotope experiments revealed dual nitrogen sources for CPANs: over 50% of nitrogen was derived from chloramine via aldehyde intermediates (phenylacetaldehyde/indole-3-acetaldehyde), while decarboxylation pathways contributed the remaining residual nitrogen from amino acid backbones. Notably, chloramine dosage governed pathway competition: Increasing chloramine concentrations (C[ArAAs]:C[NH2Cl] ≤ 1:10) progressively shift pathway dominance toward the aldehyde route during CPAN formation. Molecular electrostatic potential calculations pinpointed α-amino groups (ALIE: 0.30∼0.46 Hartree; ESP: −0.03∼0.01 Hartree) and aromatic rings as primary reactive sites, driving sequential chlorination and cyclization. These findings challenge the perception of chloramination as a "safer" alternative, emphasizing the need for precursor-specific control strategies to mitigate CPAN risks in nitrogen-rich waters.
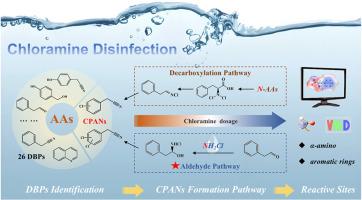
氯胺消毒中芳香族氨基酸形成氯苯乙腈的机理
氯苯基乙腈(CPANs)是一类具有高细胞毒性的氮消毒副产物(DBPs),在氯胺化水系统中被越来越多地检测到。然而,它们的形成机制,芳香族氨基酸(ArAAs),蛋白质水的关键富氮前体,仍然知之甚少。本研究系统地研究了氯胺消毒下苯丙氨酸(Phe)、酪氨酸(Tyr)和色氨酸(Trp)形成CPANs的中间体、活性位点、氮源和途径。共鉴定出26种dbp,包括7种cpan,其中色带的多样性最高(18种)。15N同位素实验揭示了CPANs的双重氮源:超过50%的氮来自氯胺,通过醛中间体(苯乙醛/吲哚-3-乙醛),而脱羧途径贡献了氨基酸主干的剩余氮。值得注意的是,氯胺剂量控制通路竞争:在CPAN形成过程中,氯胺浓度的增加(C[ArAAs]:C[NH2Cl]≤1:10)逐渐将通路优势转向醛途径。分子静电势计算确定α-氨基(ALIE: 0.30 ~ 0.46 Hartree; ESP:−0.03 ~ 0.01 Hartree)和芳香环是主要的反应位点,驱动了连续的氯化和环化。这些发现挑战了氯胺化是一种“更安全”的替代方案的看法,强调需要针对前体的控制策略来减轻富氮水域中CPAN的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


