Increased S-adenosyl methionine strengthens the suppression in mitochondrial unfolded protein response induced by 6-PPD quinone at environmentally relevant concentrations in Caenorhabditis elegans
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Exposure to 6-PPD quinone (6-PPDQ) caused mitochondrial dysfunction; however, underlying mechanisms remain largely unclear. In cells, S-adenosylmethionine (SAM) can be generated from methionine. In nematodes, 6-PPDQ (0.1-10 μg/L) reduced methionine content by decreasing expression of msra-1 encoding methionine sulfoxide reductase. 6-PPDQ further increased SAM content by enhancing expressions of sams-1 and sams-5 encoding methionine adenosyltransferases, which activated expressions of mitochondrial slc-25A26 encoding SAM transporter and trmt-10C.2 encoding tRNA methyltransferase. The 6-PPDQ induced mitochondrial dysfunction was inhibited by slc-25A26 and trmt-10C.2 RNAi. Additionally, slc-25A26 and trmt-10C.2 RNAi inhibited 6-PPDQ caused suppression in mitochondrial unfolded protein response (mt UPR) by increasing expressions of haf-1 and clpp-1, two mitochondrial genes governing mt UPR. Moreover, after treatment with methionine to reduce SAM content, 6-PPDQ induced mitochondrial dysfunction and suppression in mt UPR were inhibited. Therefore, 6-PPDQ caused increase in SAM could strengthen mitochondrial dysfunction by enhancing mt UPR suppression, which suggested a metabolic regulatory mechanism of 6-PPDQ toxicity on mitochondrial function.
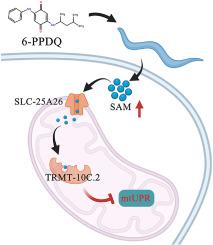
增加的s -腺苷蛋氨酸增强了环境相关浓度6-PPD醌诱导的秀丽隐杆线虫线粒体未折叠蛋白反应的抑制
暴露于6-PPD醌(6-PPDQ)引起线粒体功能障碍;然而,潜在的机制在很大程度上仍不清楚。在细胞中,s -腺苷蛋氨酸(SAM)可由蛋氨酸生成。在线虫中,6-PPDQ (0.1 ~ 10 μg/L)通过降低编码蛋氨酸亚砜还原酶msra-1的表达来降低蛋氨酸含量。6-PPDQ通过增强编码蛋氨酸腺苷转移酶的sams-1和sams-5的表达,激活编码SAM转运蛋白的线粒体slc-25A26和trmt-10C的表达,进一步增加SAM含量。2编码tRNA甲基转移酶。slc-25A26和trmt-10C.2可抑制6-PPDQ诱导的线粒体功能障碍RNAi。此外,slc-25A26和trmt-10C.2RNAi通过增加控制线粒体未折叠蛋白反应的两个线粒体基因half -1和clpp-1的表达,抑制6-PPDQ引起的线粒体未折叠蛋白反应(mt UPR)的抑制。此外,用蛋氨酸降低SAM含量后,6-PPDQ诱导的线粒体功能障碍和mt UPR的抑制被抑制。因此,6-PPDQ引起的SAM增加可能通过增强mt UPR抑制而强化线粒体功能障碍,提示6-PPDQ毒性对线粒体功能的代谢调节机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


