Valorization of vanadium industry waste into calcium-rich hydroxyapatite for ultrahigh-capacity lead remediation: A multimechanistic approach to lead sequestration
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Lead contamination poses a severe threat to ecosystems and human health; however, conventional remediation technologies are limited by inefficiency, high costs, and secondary pollution risks. Herein, present a sustainable strategy to transform vanadium industry waste—neutralization slag (Ns) into calcium-rich hydroxyapatite (Ns-HAP) for ultrahigh-capacity lead sequestration. Leveraging the abundant calcium sulfate matrix in Ns, Ns-HAP was synthesized via phosphate modification and hydrothermal crystallization, achieving a Ca/P ratio of 1.90 to enhance ion-exchange sites and reduce phosphorus consumption. Multi-technique characterization (XRD, SEM-EDS, XPS, Raman) revealed that Pb2+ immobilization by Ns-HAP operates via synergistic mechanisms: rapid surface complexation with hydroxyl/phosphate groups, ion exchange (Ca2+ and Pb2+), and covalent Pb–O–P bonding via lattice substitution, culminating in the formation of stable hydroxypyromorphite (Pb10(PO4)6(OH)2). The adsorbent exhibited an exceptional maximum Langmuir capacity of 2165.12 mg·g−1, surpassing most reported hydroxyapatite-based materials, while maintaining a relatively high Pb2+ selectivity in multi-ion systems (Cd2+, Ni2+). Thermodynamic analyses confirmed spontaneous (ΔG° = −9.88 kJ·mol−1) and endothermic (ΔH° = 50.92 kJ·mol−1) chemisorption, with kinetics governed by reaction-controlled lattice diffusion. Crucially, Ns-HAP demonstrated robust performance in high-concentration wastewater (6000 mg·L−1 Pb2+), achieving >99 % removal efficiency at an optimal dosage of 4 g·L−1. This work establishes a closed-loop paradigm for valorizing industrial waste into high-value adsorbents while elucidating mechanistic insights into Ns-HAP for advancing heavy metal remediation technologies aligned with circular economy principles.
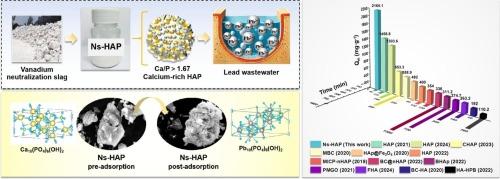
钒工业废物转化为富钙羟基磷灰石用于超高容量铅修复:一种多机制的铅隔离方法
铅污染对生态系统和人类健康构成严重威胁;然而,传统的修复技术存在效率低、成本高和二次污染风险等问题。本文提出了一种将钒工业废中和渣(Ns)转化为富钙羟基磷灰石(Ns- hap)用于超高容量铅封存的可持续策略。利用Ns中丰富的硫酸钙基质,通过磷酸盐改性和水热结晶合成Ns- hap, Ca/P比达到1.90,提高了离子交换位,降低了磷的消耗。多技术表征(XRD, SEM-EDS, XPS, Raman)表明,Ns-HAP固定Pb2+通过协同机制起作用:与羟基/磷酸基团的快速表面络合,离子交换(Ca2+和Pb2+),通过晶格取代形成共价Pb-O-P键,最终形成稳定的羟基磷闪石(Pb10(PO4)6(OH)2)。该吸附剂的最大Langmuir容量为2165.12 mg·g−1,超过了大多数羟基磷灰石基材料,同时在多离子体系(Cd2+, Ni2+)中保持了较高的Pb2+选择性。热力学分析证实了自发(ΔG° = −9.88 kJ·mol−1)和吸热(ΔH° = 50.92 kJ·mol−1)化学吸附,动力学受反应控制的晶格扩散控制。关键是,Ns-HAP在高浓度废水(6000 mg·L−1 Pb2+)中表现出强劲的性能,在最佳投加量为4 g·L−1时,去除率达到了>;99 %。这项工作建立了一个闭环范例,用于将工业废物转化为高价值吸附剂,同时阐明了Ns-HAP的机制见解,以推进符合循环经济原则的重金属修复技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


