Cryo-EM structure of endogenous Pfs230:Pfs48/45 complex with six antibodies reveals mechanisms of malaria transmission-blocking activity
IF 26.3
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
The Pfs230:Pfs48/45 complex forms the basis for leading malaria transmission-blocking vaccine candidates, yet little is known about its molecular assembly. Here, we used cryo-electron microscopy to elucidate the structure of the endogenous Pfs230:Pfs48/45 complex bound to six transmission-blocking antibodies. Our structure revealed that Pfs230 consists of multiple domain clusters rigidified by interactions mediated through insertion domains. Membrane-anchored Pfs48/45 formed a disk-like structure, interacting with a short C-terminal peptide on Pfs230 that was critical for Pfs230 membrane-retention in vivo. Membrane retention through this interaction was not essential for transmission to mosquitoes, suggesting that complex disruption is not a mode of action for transmission-blocking antibodies. Analyses of Pfs48/45- and Pfs230-targeted antibodies identified conserved epitopes on the Pfs230:Pfs48/45 complex and provided a structural paradigm for complement-dependent activity of Pfs230-targeting antibodies. Altogether, the antibody-bound Pfs230:Pfs48/45 structure improves our molecular understanding of this biological complex, informing the development of next-generation Plasmodium falciparum transmission-blocking interventions.
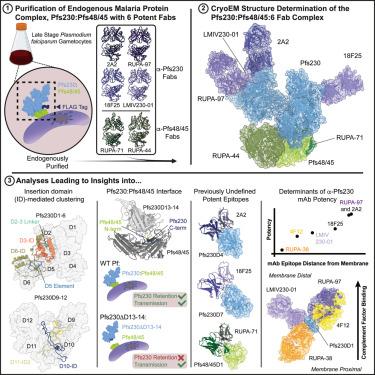
内源性Pfs230:Pfs48/45复合物与六种抗体的低温电镜结构揭示了疟疾传播阻断活性的机制
Pfs230:Pfs48/45复合物构成了主要的疟疾传播阻断候选疫苗的基础,但对其分子组装知之甚少。在这里,我们使用冷冻电子显微镜来阐明内源性Pfs230:Pfs48/45复合物与六种传输阻断抗体结合的结构。我们的结构揭示了Pfs230由多个由插入结构域介导的相互作用固化的结构域簇组成。膜锚定的Pfs48/45形成圆盘状结构,与Pfs230上的短c端肽相互作用,这对Pfs230在体内的膜保留至关重要。通过这种相互作用的膜保留对于传播给蚊子不是必需的,这表明复杂的破坏不是传播阻断抗体的作用模式。对Pfs48/45和Pfs230靶向抗体的分析发现了Pfs230:Pfs48/45复合物上的保守表位,并为Pfs230靶向抗体的补体依赖性活性提供了结构范例。总之,抗体结合的Pfs230:Pfs48/45结构提高了我们对这种生物复合体的分子理解,为下一代恶性疟原虫传播阻断干预措施的开发提供了信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


