Effect of Sc decoration and N-doping on the hydrogen storage capacity of 2D Irida-graphene monolayer: first-principles investigation
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A novel graphene allotrope of Irida-graphene (IG) with metallic property has a promising medium for hydrogen storage. This study has investigated the hydrogen storage potential of scandium-decorated IG (Sc-IG) and its nitrogen-doped counterpart (Sc-NIG) by using first-principles calculations and ab initio molecular dynamics (AIMD) simulations. The Sc-IG system exhibits half-metallic characteristics. The binding energy of Sc on IG monolayer can reach up to −3.49 eV, and five H2 molecules can be adsorbed around each Sc atom with the adsorption energy of −0.224 eV/H2, achieving a hydrogen storage capacity of 7.95 wt%. N-doping further enhances adsorption of H2 molecules. For N-doped IG with Sc decoration, a Sc atom can adsorb up to seven H2 molecules with the adsorption energy ranging from −0.198 to −0.308 eV/H2. The gravimetric density of the Sc-NIG system can reach 9.31 wt%. Analysis of electronic properties reveals that H2 adsorption proceeds primarily by Kubas-type interactions and charge polarization mechanisms. AIMD simulations confirm the structural stability of Sc-IG at 700 K, which is higher than the estimated H2 desorption temperature, indicating its potential application for reversible hydrogen storage.
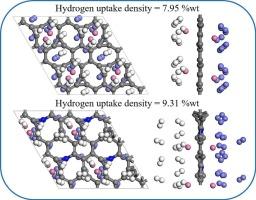
Sc修饰和n掺杂对二维铱-石墨烯单层储氢能力的影响:第一性原理研究
一种新型的具有金属性质的烯丙烯烯同素异形体(IG)是一种很有前途的储氢介质。本研究利用第一性原理计算和从头算分子动力学(AIMD)模拟研究了钪修饰IG (Sc-IG)和氮掺杂IG (Sc-NIG)的储氢潜力。Sc-IG体系具有半金属性质。Sc在IG单层上的结合能可达−3.49 eV,每个Sc原子周围可吸附5个H2分子,吸附能为−0.224 eV/H2,储氢容量为7.95 wt%。n掺杂进一步增强了H2分子的吸附。对于Sc修饰的n掺杂IG,一个Sc原子可以吸附最多7个H2分子,吸附能在−0.198 ~−0.308 eV/H2之间。Sc-NIG体系的重量密度可达9.31 wt%。电子性质分析表明,H2的吸附主要通过kubas型相互作用和电荷极化机制进行。AIMD模拟证实了Sc-IG在700 K下的结构稳定性,该温度高于预估的H2解吸温度,表明其在可逆储氢方面的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


