Ionizable Lipid Nanoparticles for mRNA Delivery: Internal Self-Assembled Inverse Mesophase Structure and Endosomal Escape
IF 17.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The clinical use of mRNA COVID-19 vaccines developed by Moderna and Pfizer-BioNTech has highlighted the critical role of ionizable lipid nanoparticles (LNPs) in the efficient loading, intracellular delivery, and cytoplasmic release of mRNAs. These LNPs typically comprise an ionizable lipid, a helper lipid, cholesterol, and a PEGylated-lipid, each contributing to the stability, structure, encapsulation efficiency, and nanoparticle–biology interactions of the final mRNA–LNPs both in vitro and in vivo. Notably, the ionizable amino-lipids, ALC-0315 used in the BioNTech/Pfizer vaccine and SM-102 in the Moderna vaccine, possess similar molecular structures, featuring multiple saturated aliphatic chains linked to a tertiary amine group via ester bonds. The acidification-induced ionization behavior of these amino-lipids is essential for enabling endosomal escape and facilitating the intracellular transfection of therapeutic mRNAs. However, despite their widespread clinical use, the physicochemical property–biological interaction and function relationships for LNPs remain poorly understood, particularly regarding how the internal nanostructural evolution during endosomal maturation influences mRNA release, endosomal escape, and gene expression. The rudimentary understanding continues to impede the rational design and optimization of RNA therapeutics.
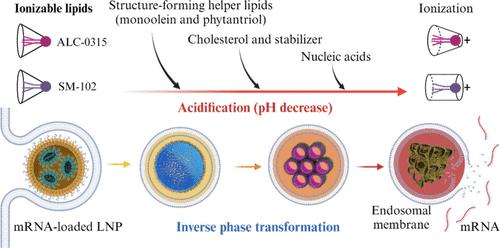
Moderna和Pfizer-BioNTech开发的mRNA COVID-19疫苗的临床应用突出了可电离脂质纳米颗粒(LNPs)在mRNA的有效装载、细胞内递送和细胞质释放中的关键作用。这些LNPs通常包括可电离脂质、辅助脂质、胆固醇和聚乙二醇化脂质,每一种都有助于最终mRNA-LNPs在体外和体内的稳定性、结构、包封效率和纳米粒子-生物学相互作用。值得注意的是,BioNTech/Pfizer疫苗中使用的ALC-0315和Moderna疫苗中使用的SM-102这两种可电离的氨基脂质具有相似的分子结构,具有多个饱和脂肪链通过酯键与叔胺基团相连。这些氨基脂质的酸化诱导电离行为对于使内体逃逸和促进治疗性mrna的细胞内转染是必不可少的。然而,尽管LNPs在临床上广泛使用,但其物理化学性质-生物相互作用和功能关系仍然知之甚少,特别是关于内体成熟过程中内部纳米结构进化如何影响mRNA释放、内体逃逸和基因表达。这种初步的认识继续阻碍着RNA疗法的合理设计和优化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Accounts of Chemical Research
化学-化学综合
CiteScore
31.40
自引率
1.10%
发文量
312
审稿时长
2 months
期刊介绍:
Accounts of Chemical Research presents short, concise and critical articles offering easy-to-read overviews of basic research and applications in all areas of chemistry and biochemistry. These short reviews focus on research from the author’s own laboratory and are designed to teach the reader about a research project. In addition, Accounts of Chemical Research publishes commentaries that give an informed opinion on a current research problem. Special Issues online are devoted to a single topic of unusual activity and significance.
Accounts of Chemical Research replaces the traditional article abstract with an article "Conspectus." These entries synopsize the research affording the reader a closer look at the content and significance of an article. Through this provision of a more detailed description of the article contents, the Conspectus enhances the article's discoverability by search engines and the exposure for the research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


