A conserved adaptor orchestrates co-secretion of synergistic type VI effectors in gut Bacteroidota
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Interbacterial competition is crucial for shaping microbial communities and is often mediated by type VI secretion systems (T6SSs) that inject effectors into competing bacteria. T6SS effectors are released via structural proteins such as VgrG, but the secretion timing and coordination are unclear. Here, we report two effectors, BtpeA (Bacteroides T6SS phosphatase effector A) and BtaeB (Bacteroides T6SS amidase effector B), within the Bacteroidota T6SS that exert distinct cell-wall destructive activities critical for interspecies competition but whose secretion is interdependent. BtpeA and BtaeB co-secretion requires an adaptor protein, BtapC (Bacteroides T6SS adaptor protein C), that mediates the sequential assembly of the pre-firing complex, VgrG-BtpeA-BtaeB-BtapC. Structural analyses of this quaternary complex elucidate multi-cargo loading mechanisms with a conserved loop in BtaeB serving as a “checkpoint” to ensure BtpeA co-secretion. During mouse colonization, the combined activities of BtpeA and BtaeB significantly exceed the sum of the individual effectors. These findings unveil a T6SS-mediated co-delivery mechanism that ensures functional synergism of effectors, highlighting potential applications in modulating gut microbiota.
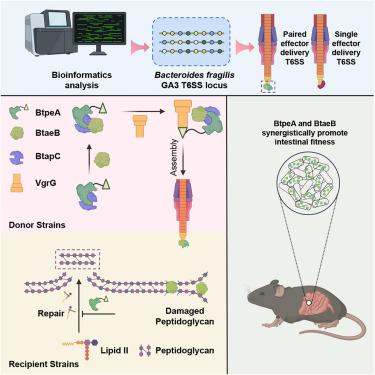
在肠道拟杆菌群中,一个保守的接头协调协同VI型效应物的共同分泌
细菌间竞争对微生物群落的形成至关重要,通常由VI型分泌系统(t6ss)介导,该系统将效应剂注入竞争细菌中。T6SS效应物通过VgrG等结构蛋白释放,但其分泌的时间和协调性尚不清楚。在这里,我们报道了两种效应物,BtpeA(拟杆菌T6SS磷酸酶效应物A)和BtaeB(拟杆菌T6SS酰胺酶效应物B),它们在拟杆菌T6SS中发挥不同的细胞壁破坏活性,对种间竞争至关重要,但它们的分泌是相互依赖的。BtpeA和BtaeB的共同分泌需要一个接头蛋白BtapC(拟杆菌T6SS接头蛋白C),该蛋白介导预点燃复合物VgrG-BtpeA-BtaeB-BtapC的顺序组装。该四元复合物的结构分析阐明了BtaeB中的一个保守环作为“检查点”以确保BtpeA共同分泌的多货加载机制。在小鼠定殖过程中,BtpeA和BtaeB的联合活性显著超过单个效应物的总和。这些发现揭示了t6ss介导的共递送机制,确保了效应物的功能协同作用,突出了在调节肠道微生物群方面的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


